Abstract
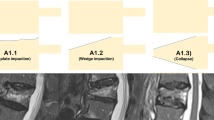
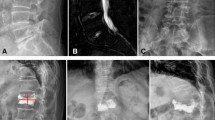
Bone consolidation is increasingly used in the treatment of both benign and malignant bone conditions. Percutaneous vertebroplasty, for example, has been shown to be useful in vertebral compression fractures in the VAPOUR trial which showed its superiority to placebo for pain reduction in the treatment of acute vertebral compressive fractures. Further tools have since been developed, such as kyphoplasty, spinal implants, and even developments in bone cements itself in attempt to improve outcome, such as chemotherapy-loaded cement or cement replacements such as radio-opaque silicon polymer. More importantly, bone fixation and its combination with cement have been increasingly performed to improve outcome. Interventional radiologists must first know the tools available, before they can best plan for their patients. This review article will focus on the tool box available for the modern interventional radiologist.







Similar content being viewed by others
References
Nussbaum DA, Gailloud P, Murphy K. The chemistry of acrylic bone cements and implications for clinical use in image-guided therapy. J Vasc Interv Radiol. 2004;15(2 Pt 1):121–6.
Arora M, et al. Polymethylmethacrylate bone cements and additives: a review of the literature. World J Orthop. 2013;4(2):67–74.
Acrylic cement in orthopaedic surgery. By JOHN CHARNLEY, C.B.E., D.Sc., F.R.C.S., Consultant Orthopaedic Surgeon, Centre for Hip Surgery, Wrighton Hospital, near Wigan. 10 × 7 1/2 in. Pp. 131 + vi, with 77 illustrations. 1970. Edinburgh: E. × S. Livingstone Ltd. 60s. British Journal of Surgery, 2005. 57(11): p. 874–874.
Phull SS, et al. Bone cement as a local chemotherapeutic drug delivery carrier in orthopedic oncology: a review. J Bone Oncol. 2021;26: 100345.
Bistolfi, A., et al., PMMA-based bone cements and the problem of joint arthroplasty infections: status and new perspectives. Materials (Basel), 2019. 12(23).
Cazzato RL, et al. Percutaneous radiofrequency ablation of painful spinal metastasis: A systematic literature assessment of analgesia and safety. Int J Hyperthermia. 2018;34(8):1272–81.
Deschamps F, et al. Cementoplasty of metastases of the proximal femur: is it a safe palliative option? J Vasc Interv Radiol. 2012;23(10):1311–6.
Cazzato RL, et al. Percutaneous long bone cementoplasty for palliation of malignant lesions of the limbs: a systematic review. Cardiovasc Intervent Radiol. 2015;38(6):1563–72.
Baroud G, et al. Load shift of the intervertebral disc after a vertebroplasty: a finite-element study. Eur Spine J. 2003;12(4):421–6.
Kim J-M, et al. Effect of bone cement volume and stiffness on occurrences of adjacent vertebral fractures after vertebroplasty. J Korean Neurosurg Soc. 2012;52(5):435.
Marcia S, et al. Effectiveness of a bone substitute (CERAMENT™) as an alternative to PMMA in percutaneous vertebroplasty: 1-year follow-up on clinical outcome. Eur Spine J. 2012;21(Suppl 1):S112–8.
Blasco J, et al. Effect of vertebroplasty on pain relief, quality of life, and the incidence of new vertebral fractures: a 12-month randomized follow-up, controlled trial. J Bone Miner Res. 2012;27(5):1159–66.
Hierholzer J, et al. Incidence of symptomatic vertebral fractures in patients after percutaneous vertebroplasty. Cardiovasc Intervent Radiol. 2008;31(6):1178–83.
Li Y-A, et al. Subsequent vertebral fracture after vertebroplasty: incidence and analysis of risk factors. Spine. 2012;37(3):179–83.
Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol. 2006;27(1):217–23.
Fribourg D, et al. Incidence of subsequent vertebral fracture after kyphoplasty. Spine. 2004;29(20):2270–6.
Tseng YY, et al. Repeated and multiple new vertebral compression fractures after percutaneous transpedicular vertebroplasty. Spine Phila Pa (1976). 2009;34(18):1917–22.
Filippiadis DK, et al. Percutaneous vertebroplasty and kyphoplasty: current status, new developments and old controversies. Cardiovasc Intervent Radiol. 2017;40(12):1815–23.
Ginebra M-P, et al. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev. 2012;64(12):1090–110.
Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. J Control Release. 2006;113(2):102–10.
Şahin, E., Calcium phosphate bone cements, in Cement Based Materials, H.E.-D.M. Saleh and R.O.A. Rahman, Editors. 2018, InTech.
Lim T-H, et al. Biomechanical evaluation of an injectable calcium phosphate cement for vertebroplasty. Spine. 2002;27(12):1297–302.
Grafe IA, et al. Calcium-phosphate and polymethylmethacrylate cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine. 2008;33(11):1284–90.
Espanol M, et al. Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater. 2009;5(7):2752–62.
Schulte TL, et al. Biomechanical comparison of vertebral augmentation with silicone and PMMA cement and two filling grades. Eur Spine J. 2013;22(12):2695–701.
Gasbarrini A, et al. Elastoplasty as a promising novel technique: vertebral augmentation with an elastic silicone-based polymer. Acta Orthop Traumatol Turc. 2017;51(3):209–14.
Bornemann R, et al. Elastoplasty: a silicon polymer as a new filling material for kyphoplasty in comparison to PMMA. Pain Physician. 2016;19(6):E885–92.
Perry CR, Pearson RL. Local antibiotic delivery in the treatment of bone and joint infections. Clin Orthop Relat Res. 1991;263:215–26.
Slane J, Gietman B, Squire M, Antibiotic elution from acrylic bone cement loaded with high doses of tobramycin and vancomycin: antibiotic elution from acrylic bone cement. J Orthopaedic Res, 2017.
Morejón Alonso L, et al. Evaluation of acrylic bone cements with single and combined antibiotics: release behavior and <i>in vitro</i> antibacterial effectiveness. Int J Polym Mater Polym Biomater. 2018;67(14):830–8.
Chen L, et al. Fabrication of the antibiotic-releasing gelatin/PMMA bone cement. Colloids Surf, B. 2019;183: 110448.
Wekwejt M, et al. Antibacterial activity and cytocompatibility of bone cement enriched with antibiotic, nanosilver, and nanocopper for bone regeneration. Nanomaterials. 2019;9(8):1114.
Slane J, et al. Mechanical, material, and antimicrobial properties of acrylic bone cement impregnated with silver nanoparticles. Mater Sci Eng C. 2015;48:188–96.
Prokopovich P, et al, A novel bone cement impregnated with silver–tiopronin nanoparticles: its antimicrobial, cytotoxic, and mechanical properties. Int J Nanomed, 2013: 2227
Prokopovich P, et al. Potent antimicrobial activity of bone cement encapsulating silver nanoparticles capped with oleic acid. J Biomed Mater Res B Appl Biomater. 2015;103(2):273–81.
Subbiahdoss G, et al. Microbial biofilm growth vs. tissue integration: “The race for the surface” experimentally studied. Acta Biomater. 2009;5(5):1399–404.
Serbetci K, Korkusuz F, Hasirci N. Thermal and mechanical properties of hydroxyapatite impregnated acrylic bone cements. Polym Testing. 2004;23(2):145–55.
Mousa WF, et al. Biological and mechanical properties of PMMA-based bioactive bone cements. Biomaterials. 2000;21(21):2137–46.
Miola M, et al. Antibacterial and bioactive composite bone cements containing surface silver-doped glass particles. Biomed Mater. 2015;10(5): 055014.
Miola M, et al. Antibiotic-free composite bone cements with antibacterial and bioactive properties. A preliminary study. Mater Sci Eng C. 2014;43:65–75.
Miola M, et al. Composites bone cements with different viscosities loaded with a bioactive and antibacterial glass. J Mater Sci. 2017;52(9):5133–46.
Verné E, et al. Antibacterial and bioactive composite bone cements. Curr Mater Sci. 2020;12(2):144–53.
Kim H, et al. The cytotoxic effect of methotrexate loaded bone cement on osteosarcoma cell lines. Int Orthop. 2001;25(6):343–8.
Wang C, et al. Bone microstructure and regional distribution of osteoblast and osteoclast activity in the osteonecrotic femoral head. PLoS ONE. 2014;9(5): e96361.
Ozben H. Cisplatin loaded PMMA: mechanical properties, surface analysis and effects on Saos-2 cell culture. Acta Orthop Traumatol Turc. 2013;47(3):184–92.
Handal JA, et al. Evaluation of elution and mechanical properties of two injectable chemotherapeutic bone cements. Chemotherapy. 2011;57(3):268–74.
Koto K, et al. Cytotoxic effects of zoledronic acid-loaded hydroxyapatite and bone cement in malignant tumors. Oncol Lett. 2017;14(2):1648–56.
Healey JH, et al. PMMA to stabilize bone and deliver antineoplastic and antiresorptive agents. Clin Orthop Relat Res. 2003;415:S263–75.
Llombart-Blanco R, et al. Local and systemic diffusion of antineoplastic drugs following vertebroplasty using acrylic cement mixed with cisplatin or methotrexate: experimental study in pigs. Eur Spine J. 2017;26(12):3216–24.
Handal JA, et al. Method for the physical analysis of drug-bone cement composite. Clin Orthop Relat Res. 2007;459:105–9.
Handal JA, et al. Polyethylene glycol improves elution properties of polymethyl methacrylate bone cements. J Surg Res. 2015;194(1):161–6.
Decker, S., et al., Cytotoxic effect of methotrexate and its solvent on osteosarcoma cells in vitro. The Journal of Bone and Joint Surgery. British volume, 1999. 81-B(3): p. 545–551.
Tahara Y, Ishii Y. Apatite cement containing cis-diamminedichloroplatinum implanted in rabbit femur for sustained release of the anticancer drug and bone formation. J Orthop Sci. 2001;6(6):556–65.
Li D, et al. A histological evaluation on osteogenesis and resorption of methotrexate-loaded calcium phosphate cement <i>in vivo</i>. Biomed Mater. 2010;5(2): 025007.
Tsoumakidou G, et al. CIRSE guidelines on percutaneous vertebral augmentation. Cardiovasc Intervent Radiol. 2017;40(3):331–42.
Bousson V, et al. Percutaneous vertebral augmentation techniques in osteoporotic and traumatic fractures. Semin Intervent Radiol. 2018;35(4):309–23.
Chen AT, Cohen DB, Skolasky RL. Impact of nonoperative treatment, vertebroplasty, and kyphoplasty on survival and morbidity after vertebral compression fracture in the medicare population. J Bone Joint Surg. 2013;95(19):1729–36.
Edidin AA, et al. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J Bone Miner Res. 2011;26(7):1617–26.
Kurup AN, et al. Balloon-assisted osteoplasty of periacetabular tumors following percutaneous cryoablation. J Vasc Interv Radiol. 2015;26(4):588–94.
Manz D, et al. Vertebral augmentation with spinal implants: third-generation vertebroplasty. Neuroradiology. 2020;62(12):1607–15.
Muto M, et al. Percutaneous treatment of vertebral fractures. Sem Musculoskelet Radiol. 2017;21(03):349–56.
Tutton SM, et al. KAST study: the kiva system as a vertebral augmentation treatment—a safety and effectiveness trial. Spine. 2015;40(12):865–75.
Noriega DC, et al. Long-term safety and clinical performance of kyphoplasty and SpineJack® procedures in the treatment of osteoporotic vertebral compression fractures: a pilot, monocentric, investigator-initiated study. Osteoporos Int. 2019;30(3):637–45.
Noriega D, et al. A prospective, international, randomized, noninferiority study comparing an implantable titanium vertebral augmentation device versus balloon kyphoplasty in the reduction of vertebral compression fractures (SAKOS study). Spine J. 2019;19(11):1782–95.
Otten LA, et al. Comparison of balloon kyphoplasty with the new Kiva® VCF system for the treatment of vertebral compression fractures. Pain Physician. 2013;16(5):E505–12.
Krüger A, et al. Height restoration and maintenance after treating unstable osteoporotic vertebral compression fractures by cement augmentation is dependent on the cement volume used. Clin Biomech. 2013;28(7):725–30.
Krüger A, et al. Height restoration of osteoporotic vertebral compression fractures using different intravertebral reduction devices: a cadaveric study. Spine J. 2015;15(5):1092–8.
Werner CML, et al. Vertebral body stenting versus kyphoplasty for the treatment of osteoporotic vertebral compression fractures: a randomized trial. J Bone Joint Surg. 2013;95(7):577–84.
Beall D, et al. Review of vertebral augmentation: an updated meta-analysis of the effectiveness. Int J Spine Surg. 2018;12(3):295–321.
Beaty, F.M.A.S.T.C.J.H., Campbell's Oper Orthopaed. 14 ed. 2020.
Synthes, Synthes Screw Reference Chart. 2002.
Matthias Hansen, R.P. Lag screw technique. AO Surgery Reference 2010 [cited 2023.
Raymond White, M.C. ORIF - Lag screws through protection plate. AO Surgery Reference 2012 [cited 2023.
Moser JE, Kunkel KAR, Gerard PD. Pullout strength of 2.0 mm cancellous and cortical screws in synthetic bone. Vet Surg. 2017;46(8):1110–5.
Chapman JR, et al. Factors affecting the pullout strength of cancellous bone screws. J Biomech Eng. 1996;118(3):391–8.
Grewal IS, Starr AJ. What’s new in percutaneous pelvis fracture surgery? Orthop Clin North Am. 2020;51(3):317–24.
Cornelis FH, et al., Percutaneous screw fixation of pelvic bone metastases using cone-beam computed tomography navigation. Diagnostic and Interventional Imaging, 2022.
Pieske O, et al. CT-guided sacroiliac percutaneous screw placement in unstable posterior pelvic ring injuries: accuracy of screw position, injury reduction and complications in 71 patients with 136 screws. Injury. 2015;46(2):333–9.
Templeman D, et al. Proximity of iliosacral screws to neurovascular structures after internal fixation. Clin Orthop Relat Res. 1996;329:194–8.
Strobl FF, et al. Technical and clinical outcome of percutaneous CT fluoroscopy-guided screw placement in unstable injuries of the posterior pelvic ring. Skeletal Radiol. 2014;43(8):1093–100.
Gangi A, Buy X. Percutaneous bone tumor management. Semin Intervent Radiol. 2010;27(2):124–36.
Lea WB, et al. Pelvis weight-bearing ability after minimally invasive stabilizations for periacetabular lesion. J Orthop Res. 2021;39(10):2124–9.
Morris MT, et al. Biomechanical restoration of metastatic cancer-induced peri-acetabular bone defects by ablation-osteoplasty-reinforcement-internal fixation technique (AORIF): To screw or not to screw? Clin Biomech (Bristol, Avon). 2022;92: 105565.
Kaya V, et al. Biomechanical and fracture characteristics of different filling and fixation methods applied to various proximal tibial metaphyseal defect sizes in an ovine model. Clin Biomech. 2022;93: 105597.
Yee DKH, et al. Cementation: for better or worse? Interim results of a multi-centre cohort study using a fenestrated spiral blade cephalomedullary device for pertrochanteric fractures in the elderly. Arch Orthop Trauma Surg. 2020;140(12):1957–64.
Suero EM, et al. Biomechanical stability of sacroiliac screw osteosynthesis with and without cement augmentation. Injury. 2021;52(10):2707–11.
Collinge CA, Crist BD. Combined percutaneous iliosacral screw fixation with sacroplasty using resorbable calcium phosphate cement for osteoporotic pelvic fractures requiring surgery. J Orthop Trauma. 2016;30(6):e217–22.
Höch A, et al. In-screw polymethylmethacrylate-augmented sacroiliac screw for the treatment of fragility fractures of the pelvis: a prospective, observational study with 1-year follow-up. BMC Surg. 2017;17(1):132.
König MA, et al. In-screw cement augmentation for iliosacral screw fixation in posterior ring pathologies with insufficient bone stock. Eur J Trauma Emerg Surg. 2018;44(2):203–10.
Wähnert D, Raschke MJ, Fuchs T. Cement augmentation of the navigated iliosacral screw in the treatment of insufficiency fractures of the sacrum: a new method using modified implants. Int Orthop. 2013;37(6):1147–50.
Sandmann GH, et al. Balloon guided cement augmentation of iliosacral screws in the treatment of insufficiency fractures of the sacrum - description of a new method and preliminary results. Acta Chir Orthop Traumatol Cech. 2018;85(2):85–8.
Bensoussan S, et al. Percutaneous reinforced cementoplasty using spindles as a palliative option for malignant fractures of the humerus. Diagn Interv Imaging. 2022;103(7):375–7.
Chiras J, et al. Interventional radiology in bone metastases. Eur J Cancer Care. 2017;26(6): e12741.
Funding
The study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, informed consent is not required.
Consent for publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chiang, J.B., Yee, D.K.H. A Toolbox of Bone Consolidation for the Interventional Radiologist. Cardiovasc Intervent Radiol 46, 1447–1457 (2023). https://doi.org/10.1007/s00270-023-03445-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-023-03445-7