Abstract
Purpose
Several retrospective studies have shown that the antitumor efficacy of capecitabine-containing chemotherapy decreases when co-administered with a proton pump inhibitor (PPI). Although a reduction in capecitabine absorption by PPIs was proposed as the underlying mechanism, the effects of PPIs on capecitabine pharmacokinetics remain unclear. We prospectively examined the effects of rabeprazole on the pharmacokinetics of capecitabine and its metabolites.
Methods
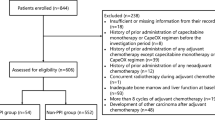
We enrolled patients administered adjuvant capecitabine plus oxaliplatin (CapeOX) for postoperative colorectal cancer (CRC) patients and metastatic CRC patients receiving CapeOX with/without bevacizumab. Patients receiving a PPI before registration were allocated to the rabeprazole group, and the PPI was changed to rabeprazole (20 mg/day) at least 1 week before the initiation of capecitabine treatment. On day 1, oral capecitabine (1000 mg/m2) was administered 1 h after rabeprazole intake. Oxaliplatin (and bevacizumab) administration on day 1 was shifted to day 2 for pharmacokinetic analysis of the first capecitabine dose. Plasma concentrations of capecitabine, 5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, and 5-fluorouracil were analyzed by high-performance liquid chromatography. Effects of rabeprazole on inhibition of cell proliferation by each capecitabine metabolite were examined with colon cancer cells (COLO205 and HCT116).
Results
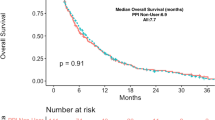
Five and 9 patients enrolled between September 2017 and July 2018 were allocated to rabeprazole and control groups, respectively. No significant effects of rabeprazole on area under the plasma concentration–time curve divided by capecitabine dose for capecitabine and its three metabolites were observed. Rabeprazole did not affect the proliferation inhibition of colon cancer cells by the respective capecitabine metabolites.
Conclusion
Rabeprazole does not affect capecitabine pharmacokinetics.



Similar content being viewed by others
References
Reigner B, Blesch K, Weidekamm E (2001) Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet 40:85–104
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34:1274–1281
Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Saltz L (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26:2006–2012
Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ (2011) Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 29:1465–1471
Schmoll HJ, Cartwright T, Tabernero J, Nowacki MP, Figer A, Maroun J, Price T, Lim R, Van Cutsem E, Park YS, McKendrick J, Topham C, Soler-Gonzalez G, de Braud F, Hill M, Sirzen F, Haller DG (2007) Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1,864 patients. J Clin Oncol 25:102–109
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, Salman P, Li J, Protsenko SA, Wainberg ZA, Buyse M, Afenjar K, Houe V, Garcia A, Kaneko T, Huang Y, Khan-Wasti S, Santillana S, Press MF, Slamon D (2016) Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—a randomized phase III trial. J Clin Oncol 34:443–451
Chu MP, Hecht JR, Slamon D, Wainberg ZA, Bang YJ, Hoff PM, Sobrero A, Qin S, Afenjar K, Houe V, King K, Koski S, Mulder K, Hiller JP, Scarfe A, Spratlin J, Huang YJ, Khan-Wasti S, Chua N, Sawyer MB (2017) Association of proton pump inhibitors and capecitabine efficacy in advanced gastroesophageal cancer: secondary analysis of the TRIO-013/LOGiC randomized clinical trial. JAMA Oncol 3:767–773
Sun J, Ilich AI, Kim CA, Chu MP, Wong GG, Ghosh S, Danilak M, Mulder KE, Spratlin JL, Chambers CR, Sawyer MB (2016) Concomitant administration of proton pump inhibitors and capecitabine is associated with increased recurrence risk in early stage colorectal cancer patients. Clin Colorectal Cancer 15:257–263
Ishizaki T, Horai Y (1999) Review article: cytochrome P450 and the metabolism of proton pump inhibitors–emphasis on rabeprazole. Aliment Pharmacol Ther 13(Suppl 3):27–36
Horai Y, Kimura M, Furuie H, Matsuguma K, Irie S, Koga Y, Nagahama T, Murakami M, Matsui T, Yao T, Urae A, Ishizaki T (2001) Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther 15:793–803
Shirai N, Furuta T, Moriyama Y, Okochi H, Kobayashi K, Takashima M, Xiao F, Kosuge K, Nakagawa K, Hanai H, Chiba K, Ohashi K, Ishizaki T (2001) Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther 15:1929–1937
Tabata T, Katoh M, Tokudome S, Hosakawa M, Chiba K, Nakajima M, Yokoi T (2004) Bioactivation of capecitabine in human liver: involvement of the cytosolic enzyme on 5′-deoxy-5-fluorocytidine formation. Drug Metab Dispos 32:762–767
De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA (1994) Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 46:594–598
de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA (1994) The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 269:15419–15422
Shimatani T, Moriwaki M, Xu J, Tazuma S, Inoue M (2006) Acid-suppressive effects of rabeprazole: comparing 10 mg and 20 mg twice daily in Japanese Helicobacter pylori-negative and -positive CYP2C19 extensive metabolisers. Dig Liver Dis 38:802–808
Wang X, Liu C, Wang J, Fan Y, Wang Z, Wang Y (2017) Proton pump inhibitors increase the chemosensitivity of patients with advanced colorectal cancer. Oncotarget 8:58801–58808
Hamzic S, Kummer D, Milesi S, Mueller D, Joerger M, Aebi S, Amstutz U, Largiader CR (2017) Novel genetic variants in carboxylesterase 1 predict severe early-onset capecitabine-related toxicity. Clin Pharmacol Ther 102:796–804
Walko CM, Lindley C (2005) Capecitabine: a review. Clin Ther 27:23–44
Wong GG, Ha V, Chu MP, Dersch-Mills D, Ghosh S, Chambers CR, Sawyer MB (2018) Effects of proton pump inhibitors on FOLFOX and CapeOx regimens in colorectal cancer. Clin Colorectal Cancer. https://doi.org/10.1016/j.clcc.2018.11.001
Hussaarts K, van Leeuwen RWF, Mathijssen RHJ (2018) Factors affecting the association of proton pump inhibitors and capecitabine efficacy in advanced gastroesophageal cancer. JAMA Oncol 4:263–264
Haruma K, Mihara M, Okamoto E, Kusunoki H, Hananoki M, Tanaka S, Yoshihara M, Sumii K, Kajiyama G (1999) Eradication of Helicobacter pylori increases gastric acidity in patients with atrophic gastritis of the corpus-evaluation of 24-h pH monitoring. Aliment Pharmacol Ther 13:155–162
Haruma K, Kamada T, Kawaguchi H, Okamoto S, Yoshihara M, Sumii K, Inoue M, Kishimoto S, Kajiyama G, Miyoshi A (2000) Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol 15:277–283
Ishizuka T, Yoshigae Y, Murayama N, Izumi T (2013) Different hydrolases involved in bioactivation of prodrug-type angiotensin receptor blockers: carboxymethylenebutenolidase and carboxylesterase 1. Drug Metab Dispos 41:1888–1895
Shi D, Yang J, Yang D, LeCluyse EL, Black C, You L, Akhlaghi F, Yan B (2006) Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J Pharmacol Exp Ther 319:1477–1484
Acknowledgements
We would like to thank Ms. Mayu Kato for her various assistance in the clinical pharmacokinetic study, and also to thank Editage for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest to declare.
Ethical approval
The study protocol was approved by the Institutional Review Board of Showa University. All patients gave written informed consent for the use of their peripheral blood samples and medical information for research purposes. The study was registered on University Hospital Medical Information Network-Clinical Trials Registry Japan (UMIN000031182).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sekido, M., Fujita, Ki., Kubota, Y. et al. Rabeprazole intake does not affect systemic exposure to capecitabine and its metabolites, 5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, and 5-fluorouracil. Cancer Chemother Pharmacol 83, 1127–1135 (2019). https://doi.org/10.1007/s00280-019-03837-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03837-y