Abstract
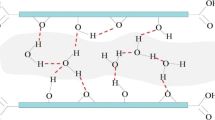
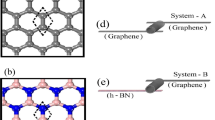
Two-dimensional (2D) materials like graphene, boron nitride, and molybdenum disulfide are utilized in diverse applications including catalysis, sensing, energy storage, biology, electronics, and so on. However, on a number of occasions, these 2D materials may not satisfy all the desired functions individually due to limitations in their properties. Thus, the urgency to generate new materials by combining and/or functionalizing these pure materials has emerged as a need of the current hour. The hybridization aids in cherishing a blend of new properties by a combination of pure materials. However, the performance of these materials in most of these applications also strongly depends on surrounding environmental factors like temperature, pressure, and especially humidity. Thus, understanding the structure of water near these hybrids and their interfaces is critical to facilitate the design of new devices with enhanced performance. Here, we have computationally designed novel layered hybrid materials of 2D sheets of graphene, boron nitride, and molybdenum disulfide by stacking them one above another. Subsequently, the structure of water in the vicinity of sheets and confined between them was studied using all-atom (AA) molecular dynamics (MD) simulations. In order to understand the dynamics of water, sheets were modeled as flexible or rigid. The structural and dynamical characteristics of hybrids and water confined between these sheets were analyzed by employing radial distribution function (RDF), average atomic-density (z-density) profile of oxygen atoms, orientational tetrahedral order (OTO), asphericity of the Voronoi cell (AVC), O–H bond orientation of water molecules, mean squared displacement (MSD), and residence time probability (Pres(t)). Based on a visual inspection and in-depth analyses on the behavior of sheets when modeled as flexible, all water molecules confined between these sheets were eliminated due to stronger interactions between sheets and their disliking toward the water. Further, in the hybrids with sheets fixed at their crystallographic positions, structural and dynamical properties of water suggest that the hydrophilic interface of BN-MoS2 followed by BN-GR and GR-MoS2 favored the stable and ordered structure of water.









Similar content being viewed by others
References
Ashby MF, Bréchet YJM (2003) Designing hybrid materials. Acta Mater 51:5801–5821
Sanchez C, Shea KJ, Kitagawa S (2011) Recent progress in hybrid materials science. Chem Soc Rev 40:471–472
Kickelbick G (2014) Hybrid materials—past, present and future. Hybrid Mater 1:39–51
Munch E, Launey ME, Alsem DH et al (2008) Tough, bio-inspired hybrid materials. Science 322:1516–1520
Le Bideau J, Viau L, Vioux A (2011) Ionogels, ionic liquid based hybrid materials. Chem Soc Rev 40:907–925
Kim S, Ku SH, Lim SY et al (2011) Graphene-biomineral hybrid materials. Adv Mater 23:2009–2014
Wang J, Malgras V, Sugahara Y, Yamauchi Y (2021) Electrochemical energy storage performance of 2D nanoarchitectured hybrid materials. Nat Commun 12:3563
Guan G, Han M-Y (2019) Functionalized hybridization of 2D nanomaterials. Adv Sci 6:1901837
Jo YK, Lee JM, Son S, Hwang S-J (2019) 2D inorganic nanosheet-based hybrid photocatalysts: Design, applications, and perspectives. J Photochem Photobiol C: Photochem Rev 40:150–190
Sinha S, Kim H, Robertson AW (2021) Preparation and application of 0D–2D nanomaterial hybrid heterostructures for energy applications. Mater Today Adv 12:100169
Perivoliotis DK, Tagmatarchis N (2017) Recent advancements in metal-based hybrid electrocatalysts supported on graphene and related 2D materials for the oxygen reduction reaction. Carbon N Y 118:493–510
Kickelbick G (2007) Hybrid Materials: synthesis, characterization, and applications. Wiley, New York
Lee SW, Cheon SA, Kim MI, Park TJ (2015) Organic-inorganic hybrid nanoflowers: types, characteristics, and future prospects. J Nanobiotechnology 13:54
Forbes JR, Gros P (2001) Divalent-metal transport by NRAMP proteins at the interface of host–pathogen interactions. Trends Microbiol 9:397–403
Guo Y, Zhang Y, Liu H et al (2010) Assembled organic/inorganic p−n junction interface and photovoltaic cell on a single nanowire. J Phys Chem Lett 1:327–330
Zhang Y, Feng X, Yuan S et al (2016) Challenges and recent advances in MOF–polymer composite membranes for gas separation. Inorg Chem Front 3:896–909
Geim AK (2009) Graphene: status and prospects. Science 324:1530–1534
Blase X, Rubio A, Louie SG, Cohen ML (1994) Stability and band gap constancy of boron nitride nanotubes. EPL 28:335
Wang J, Lee CH, Yap YK (2010) Recent advancements in boron nitride nanotubes. Nanoscale 2:2028–2034
Golberg D, Costa PMFJ, Lourie O et al (2007) Direct force measurements and kinking under elastic deformation of individual multiwalled boron nitride nanotubes. Nano Lett 7:2146–2151
Hernández E, Goze C, Bernier P, Rubio A (1998) Elastic properties of C andBxCyNzComposite nanotubes. Phys Rev Lett 80:4502–4505
Suryavanshi AP, Yu M-F, Wen J et al (2004) Elastic modulus and resonance behavior of boron nitride nanotubes. Appl Phys Lett 84:2527–2529
Chopra NG, Zettl A (1998) Measurement of the elastic modulus of a multi-wall boron nitride nanotube. Solid State Commun 105:297–300
Chang CW, Han W-Q, Zettl A (2005) Thermal conductivity of B–C–N and BN nanotubes. Appl Phys Lett 86:173102
Zettl A, Chang CW, Begtrup G (2007) A new look at thermal properties of nanotubes. Physica Status Solidi (b) 24:4181–4183
Chen Y, Zou J, Campbell SJ, Le Caer G (2004) Boron nitride nanotubes: pronounced resistance to oxidation. Appl Phys Lett 84:2430–2432
Li H, Shi Y, Chiu M-H, Li L-J (2015) Emerging energy applications of two-dimensional layered transition metal dichalcogenides. Nano Energy 18:293–305
Mattinen M, Leskelä M, Ritala M (2021) Atomic layer deposition of 2d metal dichalcogenides for electronics, catalysis, energy storage, and beyond. Adv Mater Interfaces 8:2001677
Dou M, Fyta M (2020) Lithium adsorption on 2D transition metal dichalcogenides: towards a descriptor for machine learned materials design. J Mater Chem A 8:23511–23518
Monga D, Sharma S, Shetti NP et al (2021) Advances in transition metal dichalcogenide-based two-dimensional nanomaterials. Mater Today Chem 19:100399
Li XL, Li TC, Huang S et al (2020) Controllable synthesis of two-dimensional molybdenum disulfide (MoS) for energy-storage applications. Chemsuschem 13:1379–1391
Wang H, Zhao Y, Xie Y et al (2017) Recent progress in synthesis of two-dimensional hexagonal boron nitride. J Semicond 38:031003
Sundararaju U, Mohammad Haniff MAS, Ker PJ, Menon PS (2021) MoS2/h-BN/graphene heterostructure and plasmonic effect for self-powering photodetector: A Review. Materials. https://doi.org/10.3390/ma14071672
Li X, Zhu H (2015) Two-dimensional MoS2: properties, preparation, and applications. J Materiomics 1:33–44
Bi H, Yin K, Xie X et al (2013) Ultrahigh humidity sensitivity of graphene oxide. Sci Rep 3:2714
Ghosh A, Late DJ, Panchakarla LS et al (2009) NO2and humidity sensing characteristics of few-layer graphenes. J Exp Nanosci 4:313–322
Han T, Luo Y, Wang C (2014) Effects of temperature and strain rate on the mechanical properties of hexagonal boron nitride nanosheets. J Phys Appl Phys 47:025303
Rand MJ, Roberts JF (1968) Preparation and properties of thin film boron nitride. J Electrochem Soc 115:423
Levita G, Restuccia P, Righi MC (2016) Graphene and MoS2 interacting with water: a comparison by ab initio calculations. Carbon 107:878–884
Zhao X, Perry SS (2010) The role of water in modifying friction within MoS2 sliding interfaces. ACS Appl Mater Interfaces 2:1444–1448
Melios C, Centeno A, Zurutuza A et al (2016) Effects of humidity on the electronic properties of graphene prepared by chemical vapour deposition. Carbon N Y 103:273–280
Soltani A, Thévenin P, Bakhtiar H, Bath A (2005) Humidity effects on the electrical properties of hexagonal boron nitride thin films. Thin Solid Films 471:277–286
Szmidt J (1992) Electric breakdown phenomena in C-BN layers. Diam Relat Mater 1:681–683
Brożek T, Szmidt J, Jakubowski A, Olszyna A (1994) Electrical behaviour and breakdown in plasma deposited cubic BN layers. Diam Relat Mater 3:720–724
Ronning C, Dreher E, Feldermann H et al (1997) Electrical properties and thermal stability of ion beam deposited BN thin films. Diam Relat Mater 6:1129–1134
Pritchard C, Midgley JW (1969) The effect of humidity on the friction and life of unbonded molybdenum disulphide films. Wear 13:39–50
Kwac K, Kim I, Pascal TA et al (2017) Multilayer two-dimensional water structure confined in MoS2. J Phys Chem C 121:16021–16028
Fang C, Wu X, Yang F, Qiao R (2018) Flow of quasi-two dimensional water in graphene channels. J Chem Phys 148:064702
Deshmukh SA, Kamath G, Baker GA et al (2013) The interfacial dynamics of water sandwiched between graphene sheets are governed by the slit width. Surf Sci 609:129–139
Jiao S, Duan C, Xu Z (2017) Structures and thermodynamics of water encapsulated by graphene. Sci Rep 7:2646
Lee MJ, Choi JS, Kim J-S et al (2012) Characteristics and effects of diffused water between graphene and a SiO2 substrate. Nano Res 5:710–717
Maekawa Y, Sasaoka K, Yamamoto T (2019) Comparison between microscopic structures of surficial water on hexagonal boron nitride and graphene. Appl Phys Express 12:115001
Jiang J-W, Park HS, Rabczuk T (2013) Molecular dynamics simulations of single-layer molybdenum disulphide (MoS2): Stillinger-Weber parametrization, mechanical properties, and thermal conductivity. J Appl Phys 114:064307
Xiong S, Cao G (2015) Molecular dynamics simulations of mechanical properties of monolayer MoS2. Nanotechnology 26:185705
Wagemann E, Wang Y, Das S, Mitra SK (2020) Wettability of nanostructured hexagonal boron nitride surfaces: molecular dynamics insights on the effect of wetting anisotropy. Phys Chem Chem Phys 22:2488–2497
Dutta RC, Khan S, Singh JK (2011) Wetting transition of water on graphite and boron-nitride surfaces: A molecular dynamics study. Fluid Phase Equilib 302:310–315
Raj R, Maroo SC, Wang EN (2013) Wettability of graphene. Nano Lett 13:1509–1515
Bejagam KK, Singh S, Deshmukh SA (2018) Development of non-bonded interaction parameters between graphene and water using particle swarm optimization. J Comput Chem 39:721–734
Achari PF, Bejagam KK, Singh S, Deshmukh SA (2019) Development of non-bonded interaction parameters between hexagonal boron-nitride and water. Comput Mater Sci 161:339–345
Rana MK, Chandra A (2013) Ab initio and classical molecular dynamics studies of the structural and dynamical behavior of water near a hydrophobic graphene sheet. J Chem Phys 138:204702
Gordillo MC, Martí J (2008) Structure of water adsorbed on a single graphene sheet. Phys Rev B 78:075432
Brenner DW, Shenderova OA, Harrison JA et al (2002) A second-generation reactive empirical bond order (REBO) potential energy expression for hydrocarbons. J Phys Condens Matter 14:783
Kınacı A, Haskins JB, Sevik C, Çağın T (2012) Thermal conductivity of BN-C nanostructures. Phys Rev B Condens Matter 86:115410
Jiang J-W (2015) Parametrization of Stillinger-Weber potential based on valence force field model: application to single-layer MoS2 and black phosphorus. Nanotechnology 26:315706
Wen M, Shirodkar SN, Plecháč P et al (2017) A force-matching stillinger-weber potential for MoS2: parameterization and fisher information theory based sensitivity analysis. J Appl Phys 122:244301
Rappe AK, Casewit CJ, Colwell KS et al (1992) UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114:10024–10035
Martínez L, Andrade R, Birgin EG, Martínez JM (2009) PACKMOL: a package for building initial configurations for molecular dynamics simulations. J Comput Chem 30:2157–2164
Paesani F, Zhang W, Case DA et al (2006) An accurate and simple quantum model for liquid water. J Chem Phys 125:184507
Wu Y, Tepper HL, Voth GA (2006) Flexible simple point-charge water model with improved liquid-state properties. J Chem Phys 124:024503
Sose AT, Mohammadi E, Achari PF, Deshmukh SA (2022) Determination of accurate interaction parameters between the molybdenum disulfide and water to investigate their Interfacial properties. J Phys Chem C 126:2013–2022
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J comput phys 117(1):1–19
Nosé S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81:511–519
Hoover WG (1985) Canonical dynamics: Equilibrium phase-space distributions. Phys Rev A Gen Phys 31:1695–1697
Hockney RW, Eastwood JW (2021) Computer simulation using particles. CRC Press, USA
Altabet YE, Haji-Akbari A, Debenedetti PG (2017) Effect of material flexibility on the thermodynamics and kinetics of hydrophobically induced evaporation of water. Proc Natl Acad Sci 114:E2548–E2555
Sharma S, Debenedetti PG (2012) Evaporation rate of water in hydrophobic confinement. Proc Natl Acad Sci U S A 109:4365–4370
Chow PK, Singh E, Viana BC et al (2015) Wetting of mono and few-layered WS2 and MoS2 films supported on Si/SiO2 substrates. ACS Nano 9:3023–3031
Kozbial A, Gong X, Liu H, Li L (2015) Understanding the intrinsic water wettability of molybdenum disulfide (MoS2). Langmuir 31:8429–8435
Sose AT, Cornell HD, Gibbons BJ et al (2021) Modelling drug adsorption in metal–organic frameworks: the role of solvent. RSC Adv 11:17064–17071
Narwani T, Etchebest C, Craveur P, et al (2019) In silico prediction of protein flexibility with local structure approach. arXiv [q-bio.QM]
Sun W, Du J (2019) Local ordering and interfacial structure between spinel crystal and aluminosilicate glasses from molecular dynamics simulations. Int J Appl Glass Sci 10:41–56
Wadhwa R, Yadav NS, Katiyar SP et al (2021) Molecular dynamics simulations and experimental studies reveal differential permeability of withaferin-A and withanone across the model cell membrane. Sci Rep 11:2352
Allen MP, Tildesley DJ (2017) Computer simulation of liquids. Oxford University Press, Cambridge
Frenkel D, Smit B (2001) Understanding Molecular simulation: from algorithms to applications. Elsevier, Amsterdam
Haile JM (1997) Molecular dynamics simulation: elementary methods. Wiley, New York
Giorgino T (2014) Computing 1-D atomic densities in macromolecular simulations: The density profile tool for VMD. Comput Phys Commun 185:317–322
Cicero G, Grossman JC, Schwegler E et al (2008) Water confined in nanotubes and between graphene sheets: a first principle study. J Am Chem Soc 130:1871–1878
Mosaddeghi H, Alavi S, Kowsari MH, Najafi B (2012) Simulations of structural and dynamic anisotropy in nano-confined water between parallel graphite plates. J Chem Phys 137:184703
Chau P-L, Hardwick AJ (1998) A new order parameter for tetrahedral configurations. Mol Phys 93:511–518
Galamba N (2013) Water’s structure around hydrophobic solutes and the iceberg model. J Phys Chem B 117:2153–2159
Nutt DR, Smith JC (2008) Dual function of the hydration layer around an antifreeze protein revealed by atomistic molecular dynamics simulations. J Am Chem Soc 130:13066–13073
Midya US, Bandyopadhyay S (2014) Hydration behavior at the ice-binding surface of the Tenebrio molitor antifreeze protein. J Phys Chem B 118:4743–4752
Lee SL, Debenedetti PG, Errington JR (2005) A computational study of hydration, solution structure, and dynamics in dilute carbohydrate solutions. J Chem Phys 122:204511
Giovambattista N, Rossky PJ, Debenedetti PG (2009) Effect of temperature on the structure and phase behavior of water confined by hydrophobic, hydrophilic, and heterogeneous surfaces. J Phys Chem B 113:13723–13734
Agarwal M, Kushwaha HR, Chakravarty C (2010) Local order, energy, and mobility of water molecules in the hydration shell of small peptides. J Phys Chem B 114:651–659
Malaspina DC, Schulz EP, Alarcón LM et al (2010) Structural and dynamical aspects of water in contact with a hydrophobic surface. Eur Phys J E Soft Matter 32:35–42
Elola MD, Ladanyi BM (2006) Computational study of structural and dynamical properties of formamide-water mixtures. J Chem Phys 125:184506
Bandyopadhyay D, Mohan S, Ghosh SK, Choudhury N (2014) Molecular dynamics simulation of aqueous urea solution: is urea a structure breaker? J Phys Chem B 118:11757–11768
Duboué-Dijon E, Laage D (2015) Characterization of the Local structure in liquid water by various order parameters. J Phys Chem B 119:8406–8418
Kong Z, Zhang P, Ma X et al (2020) Characterization of water structure in carbon nanotubes by various order parameters. Chem Phys 538:110887
Ruocco G, Sampoli M, Vallauri R (1992) Analysis of the network topology in liquid water and hydrogen sulphide by computer simulation. J Chem Phys 96:6167–6176
Duboué-Dijon E, Fogarty AC, Laage D (2014) Temperature dependence of hydrophobic hydration dynamics: from retardation to acceleration. J Phys Chem B 118:1574–1583
Chakraborty SN, Grzelak EM, Barnes BC et al (2012) Voronoi tessellation analysis of clathrate hydrates. J Phys Chem C 116:20040–20046
Stirnemann G, Laage D (2012) Communication: on the origin of the non-Arrhenius behavior in water reorientation dynamics. J Chem Phys 137:031101
Jedlovszky P, Pártay LB, Bartók AP et al (2008) Structural and thermodynamic properties of different phases of supercooled liquid water. J Chem Phys 128:244503
Yeh Y-L, Mou C-Y (1999) Orientational relaxation dynamics of liquid water studied by molecular dynamics simulation. J Phys Chem B 103:3699–3705
Jedlovszky P (1999) Voronoi polyhedra analysis of the local structure of water from ambient to supercritical conditions. J Chem Phys 111:5975–5985
Yan Z, Buldyrev SV, Kumar P et al (2007) Structure of the first- and second-neighbor shells of simulated water: quantitative relation to translational and orientational order. Phys Rev E 76:051201
Sciortino F, Geiger A, Stanley HE (1992) Network defects and molecular mobility in liquid water. J Chem Phys 96:3857–3865
Wikfeldt KT (2011) Structure, dynamics and thermodynamics of liquid water: insights from molecular simulations. Stockholm University, Stockholm
Maurya M, Metya AK, Singh JK, Saito S (2021) Effects of interfaces on structure and dynamics of water droplets on a graphene surface: a molecular dynamics study. J Chem Phys 154:164704
Wu M, Wei W, Liu X et al (2019) Structure and dynamic properties of stretched water in graphene nanochannels by molecular dynamics simulation: effects of stretching extent. Phys Chem Chem Phys 21:19163–19171
M R, Ayappa KG, (2019) Enhancing the dynamics of water confined between graphene oxide surfaces with janus interfaces: a molecular dynamics study. J Phys Chem B 123:2978–2993
Hamid I, Jalali H, Peeters FM, Neek-Amal M (2021) Abnormal in-plane permittivity and ferroelectricity of confined water: From sub-nanometer channels to bulk. J Chem Phys 154:114503
Smirnov KS (2017) A molecular dynamics study of the interaction of water with the external surface of silicalite-1. Phys Chem Chem Phys 19:2950–2960
Ghasemi M, Ramsheh SM, Sharma S (2018) Quantitative assessment of thermodynamic theory in elucidating the behavior of water under hydrophobic confinement. J Phys Chem B 122:12087–12096
Sharma S, Debenedetti PG (2012) Free energy barriers to evaporation of water in hydrophobic confinement. J Phys Chem B 116:13282–13289
Acknowledgements
Authors would like to acknowledge Advanced Research Computing (ARC), Virginia Tech for providing computational resources. This research used resources of the National Energy Research Scientific Computing Center (NERSC), a US Department of Energy Office of Science User Facility located at Lawrence Berkeley National Laboratory, operated under Contract No. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Additional information
Handling Editor: N. Grant Norton.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sose, A.T., Mohammadi, E., Wang, F. et al. Investigation of structure and dynamics of water confined between hybrid layered materials of graphene, boron nitride, and molybdenum disulfide. J Mater Sci 57, 10517–10534 (2022). https://doi.org/10.1007/s10853-022-07073-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07073-3