Abstract
This study aimed to evaluate the effects of pH-shifting treatment combined with microbial transglutaminase (MTG)-mediated modification on the structure, digestibility, and IgE-binding of glycinin. Glycinin was incubated in acidic (pH 1.0) or alkaline (pH 13.0) solutions to induce protein structure to unfolding followed by refolding for 1 h at pH 7.0. Afterwards, glycinin was incubated with MTG under appropriate conditions. Sodium dodecyl sulfate polyacrylamide gel electropheresis(SDS-PAGE), circular dichroism, UV absorption spectra, and surface hydrophobicity were considered to measure the changes in the structure of glycinin. The digestibility and IgE-binding of glycinin were determined by Tricine-SDS-PAGE and ELISA, respectively. The results showed that pH 1.0 shifting caused the unfolding of the spatial structure of glycinin and the formation of some polymers via disulfide bond. After glycinin was incubated with MTG, the protein preferentially underwent embedding and folding. The acidic compound-modified glycinin was stable for digestion. Under pH 13.0 shifting treatment, glycinin was partially hydrolyzed, and the MTG-modified alkaline-treated glycinin was slightly affected with a good digestibility. Compound modification could reduce the IgE-binding of glycinin, especially under alkaline conditions. Our findings suggested that alkaline pH shifting combined with MTG cross-linking can be an efficient approach to reduce the IgE-binding of glycinin with a labile digestion.
Similar content being viewed by others
Introduction
Soybean is traditionally consumed by people in Asian countries, including China, Japan, and Korea. Its consumption has increased greatly, considering as a plant source of complete proteins and a good substitute for animal proteins [1]. However, soybean is a major allergenic food for humans and animals. It was included in the list of “Big Eight” allergenic foods [2, 3]. The intake of soybean-containing food by individuals with soybean allergy can cause immunological symptoms, including urticaria, rhinitis, pruritus, asthma, anaphylactic shock, and death [4]. Approximately 0.5% of the general population, 0.4% to 3.1% of the referred population, and 12.9% of the allergic children are allergic to soybean products [5]. At least 34 allergenic proteins binding to immunoglobulin E (IgE) have been identified in soybeans and published in the Allergome Database (www.allergome.org) [6].
Glycinin, a major soybean allergen, is the predominant storage protein in soybean seeds, accounting for about 40% of the total soybean proteins [7, 8]. The protein is a hexamer oligoprotein with a molecular weight of 320-360 kDa, and it is composed of an acidic proteins (A, about 38 kDa) and a basic proteins (B, about 20 kDa) linked together via a disulfide bond [9, 10]. So far, it contains five major subunits, namely, A1aB2 (G1), A1bB1b (G2), A2B1a (G3), A3B4 (G4), and A5A4B3 (G5), and each subnits is monomeric [8]. In the formation of A-S-S-B monomer by disulfide bond (S-S) linkage, the isoelectric point of acidic and basic polypeptide chains are about 5.0 and 8.0, respectively. The acidic chain of the G1 subunit contains an IgE-binding region, which increases serum glycinin-specific IgE antibody and induces anaphylaxis [2]. The structural-functional relationship is a main determinant of the allergenicity of proteins. When native glycinin is denatured, it loses its ability to react with anti-glycinin antibodies [11, 12]. These phenomena suggest that substantial structural rearrangements occur when glycinin is denatured or disaggregated. Thus, numerous approaches, including heating [13], fermentation [14], enzymatic hydrolysate [15] and pressurization [16], have been applied to modify protein structures and to reduce or eliminate their antigenicity and potential allergenicity. Additionly, several literatures have reported the effects of in vitro digestion on the immunogenicity of soybean glycinin and/or β-conglycinin. Monci [17] demonstrated that the acid polypeptides of glycinin were more susceptible to enzymatic proteolysis than β-conglycinin in gastro-intestinal (GI) tract. Furthermore, both glycinin and β-conglycinin fragments retaining linear epitopes can trigger immune response when exposed to intestinal mucosa. So far, somestudies [18, 19] have showed that the degradation rate of basic polypeptides of glycinin was slower than that of the acidic polypeptides, so the former accounted for a large proportion of protein residues after digestion. In addition, incubation time and enzyme/substrate ratio also affect the stability and immunoreactivity of allergic proteins.
In recent years, pH-shifting is a widely employed protein modification process to improve the functionality of numerous proteins. In this process, proteins are exposed to extreme alkaline or acidic conditions to induce unfolding, and the unfolded proteins are then exposed to neutral pH to induce refolding [20, 21]. When subjected to extreme pH, proteins undergo conformational changes and form a molten globule, which is a structure that mostly retains secondary protein structure but contains a partially unfolded tertiary structure as an intermediate state [22]. Conformational changes also affect the functional properties of proteins. Roychaudhuri [23] suggested that the molten state induced by acidic conditions can affect the allergenicity of Kunitz trypsin inhibitor.
Enzymatically catalyzed cross-linking is a conventional process that modifies the function and sensory quality of food products. This process is employed to alter the biological properties of food proteins [24,25,26]. Calcium-independent microbial transglutaminase (MTG)-mediated modification can improve the function of protein. It has been reported that the solubility, emulsifying and foaming properties were improved after the protease- and acid-treated soy protein isolates (SPIs) polymerized by MTGase [27]. In addition, some studies showed that the gelling properties of soybean glycinin and β-conglycinin incresased after MTG treatment [28,29,30]. Han [31] found that all soy protein isolates except the B subunits of glycinin were cross-linked and thus form polymers. However, our previous study demonstrated that both the acidic and basic polypeptides of glycinin were effective substrates of MTG, although the reaction rate of basic chain was lower than that of acid chain [32]. Moreover, the allergenicities of peanut [33, 34], egg [25], and milk [35] were reduced after their proteins were cross-linked with MTG. Nevertheless, studies have not yet elucidated the mechanism by which MTG reduces the allergenicity of protein. Counter to this, Dekking [36] demonstrated that MTG can enhance the immunogenicity of gluten by generating T cell stimulatory epitopes involved in celiac disease. Therefore, we believe that MTG cross-linking has different effects on allergenicity of various allergic proteins.
To our knowledge, a little information was available about the relationship between structure changes and immunological properties of soybean allergens. Accordingly, we aimed to evaluate the effects of pH-shifting treatment combined with MTG-mediated modification on the structure, digestibility, and IgE-binding of glycinin. It would help to develop hypoallergenic soybean products.
Experimental
Materials
Soybean seeds (Dongnong 42) were provided by the Key laboratory of Soybean Biology (Ministry of Education), Northeast Agricultural University, China. Concanavalin A (Con A) Sepharose 4B was purchased from GE Healthcare Bio-Sciences AB (Uppsala, Sweden). Nα-CBZ-GLN-GLY, L-glutamic acidic γ-monohydroxamate, pepsin, pancreatin, bile extract, and 1-anilinonapthalene-8-sulfonate (ANS) were obtained from Sigma-Aldrich (St. Louis, Mo., USA). Commercial MTG (food additives, specific activity: 30 U/g) was purchased from Taixin Yimin Fine Chemical Industry Co., Ltd. (Taixin, China). Polyclonal rabbit anti-soybean sera were purchased from Sigma-Aldrich (St. Louis, Mo., USA). Sera containing specific IgE antibody against soybean were collected from eight patients of the First Affiliated Hospital of Guangxi Medical University in Nanning, China. The availability of sera samples was approved by the internal ethical committee of the hospital.
Preparation of Soybean Glycinin
Glycinin was isolated from soybean seeds as described previously [32]. Briefly, the dehulled and milled soybean flour was defatted for 4 h by using n-hexane (1:10, W/V), shaken, filtered, extracted twice, and air-dried. The defatted powder was dispersed in Tris-HCl buffer (50 mM, pH 8.0) containing salt (150 mM NaCl) to extract the protein. The extracts (1:15, w/v) were stirred for 4 h at 4 °C, followed by centrifugation at 9000×g for 30 min. Sodium bisulfite was added into the supernatant, and then the pH of the supernatant was adjusted to 6.4 with 1 M HCl. The supernatant was stirred for 4 h at 4 °C and centrifuged at 6500×g for 20 min. The procedure above is repeated once again. Crude glycinins were further purified using a gel filtration chromatography column Sephacryl S-300 HR and a Con A affinity column according to the method described by Hou [37]. Freeze drying was subsequently performed.
pH-Shifting Combined with MTG-Mediated Modification of Glycinin
Lyophilized glycinin powders were dispersed in 0.05 M phosphate buffer (pH 7.0) to achieve a protein concentration of 2 mg/mL by bradford method. Three groups of experiments including acidic pH (pH 1.0), neutral pH (pH 7.0) and alkaline pH (pH 13.0) were established. The glycinin solution was subsequently titrated to either a low pH of 1.0 with 4 M HCl or a high pH of 13.0 with 4 M NaOH, and the ionic strength of glycinin in phosphate solution at pH 7.0 was the same as that in acid/alkali solution. Subsequently the glycinin in the solutions were placed at room temperature for 4 h to induce partial unfolding and then neutralized to pH 7.0 to induce refolding [20]. Each sample was preheated at 50 °C for 10 min. Then, deionized water (without MTG), inactivated MTG (30 U/g) or activated MTG (30 U/g) was added to glycinin solution with pH 1.0, pH 7.0 and pH 13.0. The samples treated at pH 7.0 were termed glycinin, C-glycinin and CL-glycinin, respectively. The samples treated at pH 1.0 were termed A-glycinin, AC-glycinin, and ACL-glycinin, respectively. The samples treated at pH 13.0 were termed B-glycinin, BC-glycinin and BCL-glycinin, respectively. The route of samples preparation is shown in Fig. S1.
Structural Analysis
SDS-Page
SDS-PAGE was used to monitor the electrophoretic patterns of glycinin, whereas the modified glycinins were analyzed on the BioRad Miniprotean II system (BioRad) using 5% stacking gel and 12% separating gel. For non-reducing SDS-PAGE, the sample buffer consisted of 0.117 mol sucrose, 2 mM Tris-HCl pH 8.0, 0.03 mmol bromophenol blue and 0.35 mmol SDS, while the reducing sample buffer consisted of 2% (v/v) β-mercaptoethanol in non-reducing sample buffer. Electrophoretic bands stained with Coomassie Brilliant Blue R250 were scanned by scanning densitometer (SQ-GS800, BioRad, USA). Data were transformed into protein patterns by using Quantity One software (BioRad).
Free Sulfhydryl (SH) Group
The amount of SH group was determined by using the method described by Ellman [38]. Briefly, 1 mL of sample was added into 4 mL Tris-Gly buffer (0.086 mol Tris and 0.09 mol Gly, pH 8.0), followed by addition of 0.05 mL of 0.01 M 5,5-dithiobis-2-nitrobenzoic acid, and the mixture was subsequently incubated for 20 min in the dark at room temperature. All of the samples were measured at 412 nm. SH content was calculated based on the molar extinction coefficient of 14,150 M−1 cm−1 [39].
Circular Dichroism (CD)
We defined the conformational changes in the secondary structures by using a Far-UV CD spectroscopy (Mos-450, Biologic, Claix, France). A sample concentration of 0.2 mg/mL was used for CD spectra analysis. CD spectra were scanned in the spectral range of 190 nm to 240 nm, and the scan rate and bandwidth were set as 100 nm/min and 1.0 nm, respectively. Each sample was analyzed in triplicate, and all data were analyzed from CD spectroscopic data by the online program SELCON3 [40]. (http://dichroweb.cryst.bbk.ac.uk/html /home.shtml).
UV Absorption Spectra
UV-2450 spectrophotometer was used to measure UV absorption spectra (Shimadzu Corporation, Kyoto, Japan). The UV absorption spectra were scanned from 220 nm to 420 nm.
Surface Hydrophobicity
ANS, a hydrophobic fluorescence probe, was applied to measure surface hydrophobicity of glycinin and modified glycinin. All of the samples were dispersed in deionized water to achieve a concentration of 0.2 mg/mL. ANS (50 μL, 8 μM) was added into 5 mL protein solution. The mixture was incubated in the dark at room temperature for 1 h. Relative fluorescence was measured on a Hitachi FS F-4500 fluorescence spectrometer (Tokyo, Japan). The excitation wavelength was 375 nm, and the emission wavelength ranged from 400 nm to 600 nm at a scanning speed of 1200/min.
In Vitro Digestion
Glycinin and its cross-linked forms were digested as previously described by Amigo-Benavent [41] with a few modifications. After equilibration at 37 °C for 15 min, the pH of the samples was adjusted to 1.2 with 5 M HCl, and pepsin solution (10 mg in 5 mL of 0.1 M HCl, [E/S] = 0.02 [w/w]) was added. The pH was adjusted further to 7.5 by adding 1 M NaHCO3 dropwise, followed by continuous stirring at 37 °C for 1 h. For the intestinal digestion step, the pancreatin-bile salt mixture (10 mg of pancreatin and 60 mg of bile extract in 5 mL of 0.1 M NaHCO3, [E/S] = 0.025 [w/w]) was added into each sample. Pancreatin digestion was performed at 37 °C for 2 h under continuous stirring. The reaction was stopped by heating at 85 °C for 5 min. After the neutralization step (gastric digests) and pancreatin digestion (gastrointestinal digests), all protein samples were collected and immediately measured by Tricine-SDS-PAGE or stored at 4 °C for further analysis.
Tricine-SDS-PAGE was performed using a discontinuous system containing 16.5%, 10%, and 4% acrylamide. Loading samples were boiled at 100 °C for 5 min. The subsequent procedure was similar to that for SDS-PAGE.
Potential Allergenicity
The IgE-binding of the modified glycinin and ingested samples were estimated by inhibition ELISA as previously reported [42] with few modifications.
A 96-well ELISA microplate (plate A) was coated with 2 μg/well of native purified glycinin in carbonate buffer (0.05 M sodium carbonate, pH 9.6) overnight at 4 °C. The wells were washed thrice with PBS containing 0.05% Tween 20 (PBST), followed by blocking with 3% gelatin in PBS for 1 h at 37 °C. Another microplate (plate B) was blocked with 3% gelatin in PBS for 1 h at 37 °C after washing with PBST. For the competitive protein, 60 μL/well of modified glycinin or their corresponding gastric digests and gastrointestinal digests (5, 50, and 400 μg/mL, respectively) mixed with equal amount of the serum pool of soybean-allergic patients (diluted 1:10) were added into plate B and then incubated at 37 °C for 1 h. The mixture in plate B (100 μL/well) was transferred into plate A followed by incubation for 1 h at 37 °C. Subsequently, 100 μL of biotin-labeled goat anti-human IgE (diluted 1:2500) were added into plate A and incubated for 1 h at 37 °C. The reaction was terminated by addition of 2 M H2SO4 after incubation for 15 min. The absorbance was read with a microplate reader (BioRad) at 490 nm.
Statistical Analysis
All data were analyzed for statistical significance by using ANOVA (SPSS for Windows, version 19.0, Chicago, IL, USA). One-way analysis of variance was conducted, and differences between the sample means were performed using the Tukey test. In all cases, p < 0.05 was considered to indicate statistical significance. Samples with different letters in all histograms indicate significant differences between samples.
Results and Discussion
Effect of Compound Modification on Glycinin Structure
SDS-Page
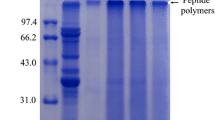
Reducing and non-reducing SDS-PAGE patterns of glycinin modified by pH-shifting and MTG cross-linking are shown in Fig. 1a and b, respectively. In the native glycinin, nearly all of the acid polypeptides were cross-linked after incubation with MTG for 2 h, whereas no significant alterations in the SDS-PAGE patterns of basic polypeptides were observed (Fig. 1a, Lane 3). This phenomenon existed because the basic polypeptide chain of native glycinin was embedded in globular molecules and cannot involve the cross-linking reaction mediated by MTG. However, most basic chain was catalytically cross-linked under acidic pH-shifting, and large polymers were present in the SDS-PAGE patterns of CL-glycinin or ACL-glycinin (Fig. 1b, Lanes 3 and 6). By contrast, glycinin was hydrolyzed to a large number of fragments under pH-shifting at pH 13.0. When the hydrolyzed fragments were cross-linked by MTG, the average molecular weight (MW) increased, whereas polymers with high MW were absent. Compared with that in the non-reducing SDS-PAGE patterns, acidic pH-shifting facilitated the formation of high-MW polymer from glycinin molecules via a disulfide bridge but did not disrupt the primary structure of glycinin. The results demonstrated that the change in the tertiary structure of molecules mainly exposed the basic chain to the MTG substrate after glycinin was subjected to acidic pH-shifting. This view is supported by our previous research [32]. Interestingly, Rabbani [22] found that Rhizopus niveus Lipase protein was partial unfolding of the protein at pH 1.8 which is in agreement with our work. Xiao [43] also found that 11S protein was higher exposure extent of the tryptophan or tyrosine-containing regions to the aqueous phase when subjected to heat treatment in acidic condition.
Surface Free Sulfhydryls
A significant increase in SH content was observed after the native glycinin was modified by MTG cross-linking. The SH contents in the modified glycinin increased when glycinin was subjected under acidic pH-shifting compared with that in the native glycinin (Fig. 2). No great change in the SH content was observed in ACL-glycinin and A-glycinin, indicating that MTG cross-linking exerts no effect on SH on the surface of glycinin treated with acidic pH-shifting.
Content of surface free sulfhydryl group in glycinin cross-linking mediated by MTG and pH-shifting. Values are expressed as averages ± standard deviation. Different characters (a to g) on the top of column indicate significant (P < 0.05). A-glycinin, AC-glycinin and ACL-glycinin were treated at pH 1, respectively. B-glycinin, BC-glycinin and BCL-glycinin were treated at pH 13, respectively
By contrast, SH contents of the modified glycinin increased sharply when glycinin was treated with akaline pH 13.0-shifting; subsequently, SH contents greatly decreased after the modified glycinin was cross-linked by MTG. This phenomenon was observed probably because akaline condition can facilitate the unfolding and hydrolysis of glycinin, resulting in cleaving the S-S bonds. Arendt [14] also concluded that free thiols were favored by the ionization of the thiol group at alkaline pH, which contributed to the content of SH increase. Furthermore, the covalent intramolecular or intermolecular cross-linking induced the refolding and aggregation of glycinin during cross-linking. Simultaneously, some free thiol groups were oxidized to form two disulfide bonds under heat. Similar results were reported by Jiang [44], who demonstrated that glycinin was considerably responsive to pH-shifting treatments, and the treatment under acidic pH solutions can increase the amount of surface SH in glycinin, leading to disulfide-mediated aggregation.
CD Analysis
The effect of pH-shifting on the secondary structure of glycinin is shown in Fig. 3a. In acidic pH-shifting treatment, an increase in random coil and a decrease in β-sheet of the secondary structure of glycinin were observed compared with the native glycinin. This trend was further observed after glycinin was treated with alkaline pH-shifting. In native glycinin or glycinin treated with acidic pH-shifting, the content of random coil in the CL-glycinin and ACL-glycinin were significantly lower than those in the cross-linked control group (C-glycinin and AC-glycinin), suggesting that MTG-catalyzed cross-linking modification can stabilize to some extent the orderly secondary structure of the native glycinin and the glycinin treated with acidic pH-shifting. In addition, compared with A-glycinin, the lower content of α-helix and the higher content of β-sheet and β-turn were observed in ACL-glycinin. This phenomenon was also observed by Zhao [45], he found that soybean protein isolates having less α-helix and β-sheet structure after glycated and cross-linked. However, the effect of MTG cross-linking on the secondary structure of glycinin treated with alkaline pH-shifting was negligible.
Far-UV CD spectroscopy and the secondary structure (a), UV absorption spectra (b) and exogeneous fluorescence spectra (c) of glycinin cross-linking mediated by MTG and pH-Shifting. A-glycinin, AC-glycinin and ACL-glycinin were treated at pH 1, respectively. B-glycinin, BC-glycinin and BCL-glycinin were treated at pH 13, respectively
Spectroscopic Analysis
The maximum UV absorption values of glycinin treated with acidic pH-shifting were significantly increased (Fig. 3b), indicating that acidic pH-shifting treatment promotes the unfolding of the tertiary structure of glycinin and the exposure of chromophore. Similar to that of native glycinin, the maximum UV absorption values of AC-glycinin or C-glycinin was greatly enhanced compared with A-glycinin or glycinin, and a slight increase in the maximum UV absorption values of ACL-glycinin or CL-glycinin was also observed, indicating that MTG cross-linking promotes the occurrence of further folding and wrapping of A-glycinin or native glycinin, rendering them with strong thermal stability. By contrast, the UV absorption values of glycinin following alkaline pH-shifting treatments were significantly reduced, as show by SDS-PAGE (Fig. 1), which was possibly caused by glycinin hydrolysis under strong alkaline pH conditions, resulting in disruption of some chromophores. Moreover, the maximum UV absorption of glycinin treated with alkaline pH-shifting was less affected by MTG cross-linking.
ANS, a hydrophobic fluorescence probe, was used to determine the hydrophobicity of compound-modified glycinin. The changes in surface hydrophobicity of glycinin modified by pH-shifting combined with MTG cross-linking were shown in Fig. 3b. The surface hydrophobicity of glycinin treated with acidic pH-shifting was considerably more enhanced than that of the native glycinin, and this phenomenon was due to the unfolding of tertiary structure and the exposure of hydrophobic groups. However, the surface hydrophobicity of ACL-glycinin decreased because of the MTG cross-linking modification of acidic pH-treated samples, and the glycinin molecule was further folded and wrapped, and the exposure of hydrophobic amino acids was reduced due to the production of covalent bonds. Moreover, the surface hydrophobicity of glycinin treated with alkaline pH-shifting decreased significantly. Actually, the surface hydrophobicity of BCL-glycinin also further decreased compared with that of B-glycinin mainly because of the partial hydrolysis of glycinin in the drastic alkaline environment of pH 13.0, and the hydrophobic groups of the molecule were destroyed. Thus, a strong hydrophobic region cannot form during MTG treatment. However, our result is different from some of previous investigations. Jiang [20] concluded that hydrophobicity increased along with the increase in alkaline pH, and extreme alkaline pH-shifting treatments were more capable of inducing conformational changes in soy protein than the acidic pH treatments.
In Vitro Digestion of Compound-Modified Glycinin
The digests of compound-modified glycinin were determined by Tricine-SDS-PAGE (Fig. 4). Native glycinin digested by gastric and Intestinal juices was almost hydrolyzed to small peptides with MW ranging from 3.4 kDa to 30 kDa. MTG-mediated cross-linking was observed to be very resistant to digestion by pepsin and pancreatin. Acidic treatment of the samples slightly affected the digestibility of native glycinin. The peptide profiles of the gastric digests (Fig. 4b) and gastrointestinal digests (Fig. 4c) of native glycinin were similar to those of A-glycinin. Moreover, these digests were hydrolyzed into small peptides after being digested by pepsin and pancreatin. The result indicated that the polymer linked S-S of glycinin induced by pH-shifiting was not stable in the digestive environment, especially under extreme pH condition of simulated digestion in the stomach. Furthermore, MTG cross-linking modification exerts the maximum effect on the digestibility of glycinin treated with acidic pH-shifting. The compound-modified glycinins demonstrated to be stable to digestion, and the fractions with a MW of greater than 100 kDa were visible even after being hydrolyzed by pepsin and pancreatin. The polymers modified with MTG were inclined to be more indigestible owing to the formation of ε-(γ-glutamyl)-lysine isopeptide bond [46, 47], thereby the structure of glycinin to be more intact via gastrointestinal digestion. Rui [48] mentioned that MTGase can form strong inter/intra molecular bonds between soy proteins which hindered digestive enzymatic hydrolysis, which is in agreement with our findings. By contrast, the glycinin treated with strong alkaline pH-shifting (pH 13.0) was partially hydrolyzed, leading to be labile to digest. Additionally, the effect of MTG cross-linking modification on the digestibility of glycinin treated by alkaline pH-shifting was minimal, whereas the digestibility of the compound modified protein (BCL-glycinin) were considerably lower than that of native glycinin.
Tricine SDS-PAGE patterns of compound modified glycinin(5 μg/mL, a), their gastric digests (60 μg/mL, b) and gastrointestinal digests (400 μg/mL, c). M: molecular weight marker; Lane 1: Glycinin; Lane 2: C-Glycinin; Lane 3: CL-Glycinin; Lane 4: A-Glycinin; Lane 5: AC-Glycinin; Lane 6: ACL-Glycinin; Lane 7: B-Glycinin; Lane 8: BC-Glycinin; Lane 9: BCL-Glycinin
Effects of Compound Modification on IgE-Binding of Glycinin
To determine the effect of compound-modified glycinin and its digests on potential allergenicity, the binding ability of glycinin to residual IgE was tested by inhibition ELISA assays. As shown in Fig. 5, the acidic pH-shifting combined with MTG cross-linking slightly influenced the IgE-binding ability of native glycinin, although it can significantly reduce the IgE-binding ability of the gastrointestinal digests. By contrast, the treatment with alkaline pH-shifting significantly reduced the IgE-binding ability of glycinin. This finding is attributed to the partial hydrolysis of glycinin under alkaline pH-shifting. Moreover, the IgE-binding of glycinin treated with the alkaline pH-shifting combined with MTG cross-linking was decreased by approximately 30%. The changes in the IgE-binding of gastric (Fig. 5b) and gastrointestinal digests (Fig. 5c) were similar to those of modified glycinin (Fig. 5a) under alkaline pH-shifting. To our knowledge, the changes in the structure of allergen, especially allergenic epitopes, are the most fundamental factors in allergenicity variation. Furthermore, the structure of a protein and its immunological properties are correlated. In fact, the result of the IgE-binding ability of glycinin were supported by our analyses on allergen structure during compound modification.
IgE-binding reactivity of modified glycinin (a), their gastric digests (b) and gastrointestinal digests (c) determined by E LISA. Values are expressed as averages ± standard deviation. Different characters on the top of column denote significant (P < 0.05) differences among samples. A-glycinin, AC-glycinin and ACL-glycinin were treated at pH 1, respectively. B-glycinin, BC-glycinin and BCL-glycinin were treated at pH 13, respectively
Conclusions
Strong acidic pH-shifting can promote not only the unfolding of the spatial structure of glycinin but also the formation of high-MW polymer from glycinin molecules via a disulfide bridge. The effect of MTG cross-linking on the structure of glycinin was dominated by embedding and folding. By contrast, alkaline pH-shifting can dissociate and partially hydrolyze glycinin, and its structure was nearly unaffected by subsequent MTG cross-linking. Moreover, acidic pH-shifting combined with MTG cross-linking modification can enhance the resistance of glycinin to gastrointestinal digestion and reduce its IgE-binding to a certain extent. Compared with native glycinin, glycinin treated with alkaline pH-shifting combined with MTG cross-linking modification can significantly reduce the susceptibility of glycinin to gastrointestinal digestion and its potential allergenicity. Accordingly, alkaline pH-shifting combined with MTG cross-linking may be an efficient approach to reduce the IgE-binding of glycinin.
References
E.Y. Kim, K.B. Hong, H.J. Suh, H.S. Choi, Food Funct. 6(11), 3512–3521 (2015)
S. Hu, H. Liu, S. Qiao, P. He, X. Ma, W. Lu, J Agric Food Chem 61(18), 4406–4410 (2013)
B. Liu, D. Teng, Y.L. Yang, X.M. Wang, J.H. Wang, Process Biochem. 47(2), 280–287 (2012)
E. Penas, G. Prestamo, F. Polo, R. Gomez, Food Chem. 99(3), 569–573 (2006)
Y. Katz, P. Gutierrez-Castrellon, M.G. Gonzalez, R. Rivas, B.W. Lee, P. Alarcon, Clin Rev Allergy Immunol 46(3), 272–281 (2014)
H. Yang, A.S. Yang, J.Y. Gao, H.B. Chen, J. Food Sci. 79(11), C2157–C2163 (2014)
J. Chen, J. Wang, P. Song, X. Ma, Food Chem. 162, 27–33 (2014)
T. Wang, G.X. Qin, Z.W. Sun, Y. Zhao, Crit. Rev. Food Sci. Nutr. 54(7), 850–862 (2014)
K. Prak, B. Mikami, T. Itoh, T. Fukuda, N. Maruyama, S. Utsumi, Acta Crystallogr Sect F 69(8), 937–941 (2013)
B. Yuan, J. Ren, M. Zhao, D. Luo, L. Gu, LWT Food Sci Technol 46(2), 453–459 (2012)
L. Tuppo, R. Spadaccini, C. Alessandri, A. Mari, R. Boelens, Biopolymers 102(5), 416–425 (2014)
W. Chen, J.Y. Gao, H.B. Chen, Food Sci. 23, 391–394 (2010)
Z. Li, Y. Luo, M. Jiang, J. Aquat, Food Product Technol. 25(3), 350–357 (2015)
O.E. Makinen, E. Zannini, P. Koehler, E.K. Arendt, Food Chem. 196, 17–24 (2016)
D. Sung, K.M. Ahn, S.Y. Lim, S. Oh, J Sci Food Agric 94(12), 2482–2487 (2014)
X. Ma, D. Lozano, H. Ojalvo, R. Chen, E. Lopez Fandiño, I. Molina, Food Sci. Emerg. 29, 143–150 (2015)
L. Monaci, S.L. Bavaro, E. De Angelis, L. Monaci, Food Funct. (2017)
G.X. Qin, Z.W. Sun, Y. Zhao, Food Agric. Immunol. 21(3), 23–263 (2010)
Y. Li, L.Z, Jiang, Y. Yang, R. Wang, F.F. Han, Biomed. Res. Int. (2016)
J. Jiang, J. Chen, Y.L. Xiong, J Agric Food Chem 57(16), 7576–7583 (2009)
Y. Liang, H.G. Kristinsson, Food Res. Int. 40(6), 668–678 (2007)
G. Rabbani, E. Ahmad, N. Zaidi, S. Fatima, R.H. Khan, Cell Biochem. Biophys. 62(3), 487–499 (2012)
R. Roychaudhuri, G. Sarath, M. Zeece, J. Markwell, Biochem. Biophys. Acta 1699(1–2), 207–212 (2004)
Y.H. Chang, S.Y. Shiau, F.B. Chen, F.R. Lin, LWT Food Sci Technol 44(4), 1107–1112 (2011)
R. Porta, C.V.L. Giosafatto, P. di Pierro, A. Sorrentino, L. Mariniello, Amino Acids 44(1), 285–292 (2013)
C. Xia, L. Wang, Y. Dong, S. Zhang, S.Q. Shi, L. Cai, J. Li, RSC Adv. 5(101), 82765–82771 (2015)
E.F.E. Babiker, M.A.S. han, N. Matsudomi, A. Kato, Food Res. Int. 29(7), 627–634 (1996)
L.J. Kang, Y. Matsumura, K. Ikura, J Agric Food Chem 42(1), 159–165 (1994)
Y. Chanyongvorakul, Y. Matsumura, M. Nonaka, M. Motoki, T. Mori, J. Food Sci. 60(493), 483–488 (1995)
C.H. Tang, H. Wu, Z. Chen, Food Res. Int. 39(1), 87–97 (2006)
M.Y. Han, H.Z. Zu, X.L. Xu, G.H. Zhou, J Food Process Preserv 39(3), 309–317 (2015)
A.S. Yang, J.H. Xia, Y.Q. Gong, H.B. Chen, Modern Food Sci. Technol. 33(2), 55–60 (2017)
D.A. Clare, G. Gharst, S.J. Maleki, T.H. Sanders, J Agric Food Chem 56(22), 10913–10921 (2008)
Y. Zhou, J.S. Wang, X.J. Yang, D.H. Lin, Y.F. Gao, Y.J. Su, S. Yang, Y.J. Zhang, J.J. Zheng, Int. J. Food Sci. 2013, 1–8 (2013)
M.B. Villas-Boas, M.A. Fernandes, R. de Lima Zollner, F.M. Netto, Int. Dairy J. 25(2), 123–131 (2012)
E. Dekking, P. Van Veelen, A. De Ru, E. Kooy Winkelaar, T. Groneveld, W. Nieuwenhuizen, F. Koning, J. Cereal Sci. 47(2), 339–346 (2008)
D.H.J. Hou, S.K.C. Chang, J Agric Food Chem 52(12), 3792–3800 (2004)
G.L. Ellman, Arch. Biochem. Biophys. 82(1), 70–77 (1959)
P. Eyer, D. Kiderlen, F. Worek, Anal. Biochem. 312(2), 224–227 (2003)
N. Sreerama, R.W. Woody, Anal. Biochem. 287(2), 252–260 (2000)
M. Amigo Benavent, A. Clemente, P. Ferranti, S. Caira, M.D. del Castillo, Food Chem. 129(4), 1598–1605 (2011)
A.S. Yang, C.Y. Long, J.H. Xia, P. Tong, Y.F. Cheng, Y. Wang, H.B. Chen, J Sci Food Agric 97(1), 199–206 (2016)
J. Xiao, C. Shi, L. Zhang, Y. Li, J. Qi, Y. Wang, Q. Huang, Food Res. Int. 89(1), 540–548 (2016)
J. Jiang, Y.L. Xiong, M.C. Newman, G.K. Rentfrow, Food Chem. 132(4), 1944–1950 (2012)
C.Y. Zhao, H.F. Liu, M. Fu, X.H. Zhao, CyTA - J Food 14(1), 138–144 (2015)
A.L. Gaspar, S.P. de Goes Favoni, Food Chem. 171, 315–322 (2015)
P. Zhang, T. Hu, S. Feng, Q. Xu, T. Zheng, M. Zhou, X. Chu, X. Huang, X. Lu, S. Pan, E.C. Li-Chan, H. Hu, Ultrason. Sonochem. 29, 380–387 (2016)
X. Rui, Y. Fu, Q. Zhang, W. Li, F. Zare, X. Chen, M. Jiang, M. Dong, LWT Food Sci Technol 71, 234–242 (2016)
Acknowledgments
The authors are grateful for financial support of National Natural Science Foundation of China (No. 31460439, 31760453), National High Technology Research and Development Program of China (863 Program, No. 2013AA102205), Young Scientist Training Program of Jiangxi Province (No. 20122BCB23006), International Science & Technology Cooperation Program of Jiangxi Province (No. 20142BDH80002).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Flow chart of the pH-shifting combined with MTG-mediated modification of glycinin (DOCX 2694 kb)
Rights and permissions
About this article
Cite this article
Yang, A., Bai, J., Xia, J. et al. Structure Changes in Relation to Digestibility and IgE-Binding of Glycinin Induced by pH-Shifting Combined with Microbial Transglutaminase-Mediated Modification. Food Biophysics 14, 269–277 (2019). https://doi.org/10.1007/s11483-019-09580-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-019-09580-4