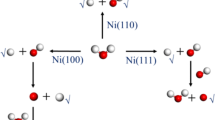
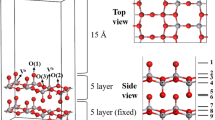
Abstract
Sea salt particles containing NaCl are among the most abundant particulate masses in coastal atmosphere. Reactions involving sea salt particles potentially generate Cl radicals, which are released into coastal atmosphere. Cl radicals play an important role in the nitrogen and O3 cycles, sulfur chemistry and particle formation in the troposphere of the polluted coastal regions. This paper aimed at the heterogeneous reaction between gaseous HNO3 and solid NaCl. The mechanism was investigated by density functional theory (DFT). The results imply that water molecules induce the surface reconstruction, which is essential for the heterogeneous reaction. The surface reconstruction on the defective (710) surface has a barrier of 10.24 kcal∙mol–1 and is endothermic by 9.69 kcal∙mol–1, whereas the reconstruction on the clean (100) surface has a barrier of 18.46 kcal$mol–1 and is endothermic by 12.96 kcal∙mol–1. The surface reconstruction involved in water-adsorbed (710) surface is more energetically favorable. In comparison, water molecules adsorbed on NaCl (100) surface likely undergo water diffusion or desorption. Further, it reveals that the coordination number of the Cl –out is reduced after the surface reconstruction, which assists Cl –out to accept the proton from HNO3. HCl is released from heterogeneous reactions between gaseous HNO3 and solid NaCl and can react with OH free radicals to produce atomic Cl radicals. The results will offer further insights into the impact of gaseous HNO3 on the air quality of the coastal areas.
Graphical abstract

Similar content being viewed by others
References
Riedel T P, Bertram T H, Crisp T A, Williams E J, Lerner B M, Vlasenko A, Li S M, Gilman J, de Gouw J, Bon D M, Wagner N L, Brown S S, Thornton J A. Nitryl chloride and molecular chlorine in the coastal marine boundary layer. Environmental Science & Technology, 2012, 46(19): 10463–10470
Chang S, Allen D T. Atmospheric chlorine chemistry in southeast texas: impacts on ozone formation and control. Environmental Science & Technology, 2006, 40(1): 251–262
Sommariva R, Glasow R. Multiphase halogen chemistry in the tropical Atlantic ocean. Environmental Science & Technology, 2012, 46(19): 10429–10437
Allan W, Struthers H, Lowe D C. Methane carbon isotope effects caused by atomic chlorine in the marine boundary layer: global model results compared with southern hemisphere measurements. Journal of Geophysical Research, D, Atmospheres, 2007, 112(D4): D04306
Keene WC, Stutz J, Pszenny A A P, Maben J R, Fischer E V, Smith A M, von Glasow R, Pechtl S, Sive B C, Varner R K. Inorganic chlorine and bromine in coastal New England air during summer. Journal of Geophysical Research, D, Atmospheres, 2007, 112(D10): D10S12
Philip S, Martin R V, van Donkelaar A, Lo J W, Wang Y, Chen D, Zhang L, Kasibhatla P S, Wang S, Zhang Q, Lu Z, Streets D G, Bittman S, Macdonald D J. Global chemical composition of ambient fine particulate matter for exposure assessment. Environmental Science & Technology, 2014, 48(22): 13060–13068
Gregory R C, Bhupesh A, Sarika K, Alessio D, Youhua T, David S, Qiang Z, Tami C B, Veerabhadran R, Aditsuda J, Pallavi M. Asian aerosols: current and year 2030 distributions and implications to human health and regional climate change. Environmental Science & Technology, 2009, 43(15): 5811–5817
Davies J A, Cox R A. Kinetics of the heterogeneous reaction of HNO3 with NaCl: effect of water vapor. Journal of Physical Chemistry A, 1998, 102(39): 7631–7642
Evans C D, Monteith D T, Fowler D, Cape J N, Brayshaw S. Hydrochloric acid: an overlooked driver of environmental change. Environmental Science & Technology, 2011, 45(5): 1887–1894
Yao X, Fang M, Chan C K. Experimental study of the sampling artifact of chloride depletion from collected sea salt aerosols. Environmental Science & Technology, 2001, 35(3): 600–605
Ro C U, Kim H, Oh K Y, Yea S K, Lee C B, Jang M, Van Grieken R. Single-particle characterization of urban aerosol particles collected in three Korean cities using low-Z electron probe X-ray microanalysis. Environmental Science & Technology, 2002, 36(22): 4770–4776
Hess M, Krieger U K, Marcolli C, Peter T, Lanford W A. Uptake of nitric acid on NaCl single crystals measured by backscattering spectrometry. Nuclear Instruments & Methods in Physics Research. Section B, Beam Interactions with Materials and Atoms, 2010, 268 (11–12): 2202–2204
Nishikawa Y, Kannari A. Atmospheric concentration of ammonia, nitrogen dioxide, nitric acid, and sulfur dioxide by passive method within Osaka prefecture and their emission inventory. Water, Air, and Soil Pollution, 2010, 215(1–4): 229–237
Fenter F F, Caloz F, Rossi MJ. Kinetics of nitric acid uptake by salt. Journal of Physical Chemistry, 1994, 98(39): 9801–9810
Finlayson-Pitts B J, Hemminger J C. Physical chemistry of airborne sea salt particles and their components. Journal of Physical Chemistry A, 2000, 104(49): 11463–11477
Ro C U, Oh K Y, Kim Y P, Lee C B, Kim K H, Kang C H, Osán J, de Hoog J, Worobiec A, Van Grieken R. Single-particle analysis of aerosols at Cheju Island, Korea, using low-Z electron probe X-ray microanalysis: a direct proof of nitrate formation from sea salts. Environmental Science & Technology, 2001, 35(22): 4487–4494
Allen H C, Laux J M, Vogt R, Finlayson-Pitts B J, Hemminger J C. Water-induced reorganization of ultrathin nitrate films on NaCl: implications for the tropospheric chemistry of sea salt particles. Journal of Physical Chemistry, 1996, 100(16): 6371–6375
Delley B. An all-electron numerical method for solving the local density functional for polyatomic molecules. Journal of Chemical Physics, 1990, 92(1): 508–517
Delley B. From molecules to solids with the DMol3 approach. Journal of Chemical Physics, 2000, 113(18): 7756–7764
Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Physical Review Letters, 1996, 77(18): 3865–3868
Rabuck A D, Scuseria G E. Performance of recently developed kinetic energy density functions for the calculation of hydrogen binding strengths and hydrogen-bonded structures. Theoretical Chemistry Accounts, 2000, 104(6): 439–444
McNellis E R, Meyer J, Reuter K. Azobenzene at coinage metal surfaces: role of dispersive van der Waals interactions. Physical Review B: Condensed Matter and Materials Physics, 2009, 80(20): 205414
Li B, Michaelides A, Scheffler M. Density functional theory study of flat and stepped NaCl(001). Physical Review B: Condensed Matter and Materials Physics, 2007, 76(7): 075401
Monkhorst H J, Pack J D. Special points for Brillouin-zone integrations. Physical Review B: Condensed Matter and Materials Physics, 1976, 13(12): 5188–5192
Govind N, Petersen M, Fitzgerald G, King-Smith D, Andzelm J. A generalized synchronous transit method for transition state location. Computational Materials Science, 2003, 28(2): 250–258
Jonsson H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. Journal of Chemical Physics, 2000, 113(22): 9978–9985
Nickels J E, Fineman M A, Wallace W E. X-Ray diffraction studies of sodium chloride-sodium bromide solid solutions. Journal of Physical Chemistry, 1949, 53(5): 625–628
Yang Y, Meng S, Wang E. Water adsorption on a NaCl (001) surface: adensity functional theory study. Physical Review B: Condensed Matter and Materials Physics, 2006, 74(24): 245409
Bruch L W, Glebov A, Toennies J P, Weiss H. A helium atom scattering study of water adsorption on the NaCl(100) single crystal surface. Journal of Chemical Physics, 1995, 103(12): 5109–5120
Folsch S, Stock A, Henzler M. Two-dimensional water condensation on the NaCl(100) surface. Surface Science, 1992, 264(1–2): 65–72
Li B, Michaelides A, Scheffler M. How strong is the bond between water and salt? Surface Science, 2008, 602(23): L135–L138
Ahlswede K J. MSINDO Study of the adsorption of water molecules at defective NaCl(100) surfaces. Surface Science, 1999, 439(1): 86–94
Verdaguer A, Sacha G M, Luna M, Ogletree D F, Salmeron M. Initial stages of water adsorption on NaCl (100) studied by scanning polarization force microscopy. Journal of Chemical Physics, 2005, 123(12): 124703
Pepa C, Sanfelix A A, George R D, Daniel S. Water adsorption and diffusion on NaCl(100). Journal of Physical Chemistry B, 2006,48 (110): 24559–24564
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhao, N., Zhang, Q. & Wang, W. Heterogeneous reaction mechanism of gaseous HNO3 with solid NaCl: a density functional theory study. Front. Environ. Sci. Eng. 10, 3 (2016). https://doi.org/10.1007/s11783-016-0836-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-016-0836-z