Abstract
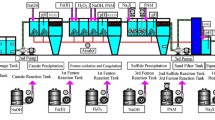
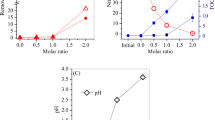
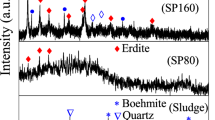
Coagulation is commonly applied to treat Zn-bearing wastewater from smelting industries (smelting wastewater), and thus the Zn-bearing sludge was considerably produced, which should be solidified before safety disposal. Herein, we demonstrated a novel approach to recycle Zn effectively from smelting wastewater via an integrated Fe coagulation and hematite precipitation method. First, smelting wastewater was coagulated by adding ferric chloride to generate Fe/Zn-bearing sludge (sludge for short). Secondly, the sludge was dissolved to generate an acid solution containing 2.2 g/L of Zn and 39.2 g/L of Fe. Thirdly, the Fe/Zn-bearing solution was hydrothermally treated, and 89% of Fe was eliminated to highly purified hematite block, whereas the percentage of Zn lost was below 1.1%. Finally, the hematite precipitates were collected, and the supernatant was hydrothermally treated again with the addition of glucose. When the molar ratio of glucose to Fe in the supernatant was 1.5, over 99.5% of Fe was precipitated in hematite nanoparticles with a diameter of 10–100 nm, and the residual Fe was 21.5 mg/L. The loss of Zn was below 0.4%, and the residual Zn in the solution was 2169 mg/L, 290 times of that in the smelting wastewater. The major mechanism for Fe removal was the hydrolysis of ferric nitrate into hematite, which was promoted by nitrate consumption in glucose oxidation. This paper is the first report of an environment-friendly method for enriching Zn without generating any waste.

Similar content being viewed by others
References
Arslan C, Arslan F (2002). Recovery of copper, cobalt, and zinc from copper smelter and converter slags. Hydrometallurgy, 67(1–3): 1–7
Binnemans K, Jones P T, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013). Recycling ofrare earths: A critical review. Journal of Cleaner Production, 51: 1–22
Charlatchka R, Cambier P (2000). Influence of reducing conditions on solubility of trace metals in contaminated soils. Water, Air, and Soil Pollution, 118(1/2): 143–168
Cox J L, Hallen R T, Lilga M A (1994). Thermochemical nitrate destruction. Environmental Science & Technology, 28(3): 423
Danil de Namor A F, El Gamouz A, Frangie S, Martinez V, Valiente L, Webb O A (2012). Turning the volume down on heavy metals using tuned diatomite: A review of diatomite and modified diatomite for the extraction of heavy metals from water. Journal of Hazardous Materials, 241–242: 14–31
Delgado-Andrade C, Seiquer I, Nieto R, Navarro M P (2004). Effects of heated glucose–lysine and glucose–methionine model-systems on mineral solubility. Food Chemistry, 87(3): 329–337
Fanning J C (2000). The chemical reduction of nitrate in aqueous solution. Coordination Chemistry Reviews, 199(1): 159–179
Fu F, Wang Q (2011). Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management, 92(3): 407–418
Geng Z, Yu Y, Zhu S, Yu H, Liu J, Bian D, Yang X, Huo H, Huo M (2017). Comparing Polyethersulfone and Polyurethane-immobilized cells of Comamonas testosteroni QYY in treatment of an accidental dye wastewater. Chemical Research in Chinese Universities, 33(1): 36–43
Ismael M R C, Carvalho J M R (2003). Iron recovery from sulphate leach liquors in zinc hydrometallurgy. Minerals Engineering, 16(1): 31–39
Jiang J Q, Graham N J (1998). Pre-polymerised inorganic coagulants and phosphorus removal by coagulation- a review. Water S.A., 24(3): 237–244
Langová Š, Matýsek D (2010). Zinc recovery from steel-making wastes by acid pressure leaching and hematite precipitation. Hydrometallurgy, 101(3–4): 171–173
Lewis A E (2010). Review of metal sulphide precipitation. Hydrometallurgy, 104(2): 222–234
Li Y C, Min X B, Chai L Y, Shi M Q, Tang C J, Wang Q W, Liang Y J, Lei J, Liyang W J (2016). Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals. Journal of Environmental Management, 181: 756–761
Liu S, Sun J, Huang Z (2010). Carbon spheres/activated carbon composite materials with high Cr(VI) adsorption capacity prepared by a hydrothermal method. Journal of Hazardous Materials, 173(1–3): 377–383
Li M, Li W, Liu S (2012). Control of the morphology and chemical properties of carbon spheres prepared from glucose by a hydrothermal method. Journal of Materials Research, 27(8): 1117–1123
Okoro H K, Fatoki O S, Adekola F A, Ximba B J, Snyman R G (2012). A review of sequential extraction procedures for heavy metals speciation in soil and sediments. Critical Reviews in Environmental Science and Technology, 40(5): 365–399
Schwertmann U, Friedl J, Stanjek H (1999). From Fe (III) ions to ferrihydrite and then to hematite. Journal of Colloid and Interface Science, 209(1): 215–223
Šubrt J, Štengl V, Skokánek M (1992). Decomposition of ferrihydrite prepared from Fe(NO3)3 aqueous solutions under varying pH. Thermochimica Acta, 211: 107–119
Tang X, Zheng H, Teng H, Sun Y, Guo J, Xie W, Yang Q, Chen W (2016). Chemical coagulation process for the removal of heavy metals from water: A review. Desalination and Water Treatment, 57 (4): 1733–1748
Yang F, Deng Z, Wei C, Li C, Li X B (2014). Iron-removal by hematite from leaching liquor of high iron sphalerite. Chin J Nonferrous Met, 24(9): 2387–2392
Ying S L (2006). Investigation of hydrolysis and coagulation mechanism of poly-iron coagulant. Chinese Journal of Geochemistry, 25(S1 B08): 149–150
Zhu S, Dong G, Yu Y, Yang J, Yang W, Fan W, Zhou D, Liu J, Zhang L, Huo M (2018). Hydrothermal synthesis of amagnetic adsorbent from wasted iron mud for effective removal of heavy metals from smelting wastewater. Environ Sci Pollut R: 1–15
Zhu S, Fang S, Huo M, Yu Y, Chen Y, Yang X, Geng Z, Wang Y, Bian D, Huo H (2015). A novel conversion of the groundwater treatment sludge to magnetic particles for the adsorption of methylene blue. Journal of Hazardous Materials, 292: 173–179
Zhu S, Lin X, Dong G, Yu Y, Yu H, Bian D, Zhang L, Yang J, Wang X, Huo M (2019). Valorization of manganese-containing groundwater treatment sludge by preparing magnetic adsorbent for Cu(II) adsorption. Journal of Environmental Management, 236: 446–454
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (Grant Nos. 51578118, 51878134, 51678273 and 51878133) and the Science and Technology Program of Jilin Province (Grant No. 20190303001SF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• 98.5% Zn was enriched from Zn-bearing smelting wastewater.
• 99.5% Fe was hydrothermally precipitated into hematite nanoparticles.
• Highly purified hematite nanoparticles were obtained.
• The residual Zn was 2169 mg/L, 290 times of that in smelting wastewater.
Rights and permissions
About this article
Cite this article
Qu, Z., Su, T., Chen, Y. et al. Effective enrichment of Zn from smelting wastewater via an integrated Fe coagulation and hematite precipitation method. Front. Environ. Sci. Eng. 13, 94 (2019). https://doi.org/10.1007/s11783-019-1178-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-019-1178-4