Abstract
Purpose of Review
Because the fetus expresses paternally-derived foreign antigens, pregnancy poses unique immune challenges. The maternal immune system must balance protecting the semi-allogeneic fetus from immune rejection with defending the mother and fetus from pathogens. Fetally-derived trophoblast cells of the placenta serve as the immunologic interface with soluble and cellular maternal immune effectors and are thereby essential partners in supporting these tightly regulated interactions. While there are multiple ways that the maternal–fetal immune interface is controlled in a healthy pregnancy, this review highlights several of the immune checkpoint regulators thought to be centrally involved in maternal–fetal immunoregulation.
Recent Findings
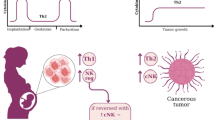
Reproductive immunologists have shown that those fetal trophoblast cells that directly encounter maternal immune cells share many common features with cancer cells, shifting the paradigm of placental immunology away from transplantation biology and toward our extensive understanding of tumorigenesis. Both the post-implantation placenta and the growing neoplasm have many shared goals, including invasion, robust cellular proliferation, angiogenesis, and modulation of host immunity. One way in which both the human placenta and cancer cells protect themselves from immune attack is through the loss of, or neoexpression of, several important cell surface regulators of specific immune interactions known as immune checkpoints. Here, we will discuss several ways that tumors and the placenta utilize immune checkpoint pathways and inhibitors, including the loss of most classical major histocompatibility complex (MHC) molecules and neoexpression of several nonclassical MHC molecules, expression of novel immunosuppressive B7 family members and cell adhesion molecules, such as CD47, and modulation of indoleamine 2,3-dioxygenase (IDO) enzyme activity.
Summary
Finely tuned immune adaptation is fundamental to a successful reproduction. Failure to implement such adaptations can result in a variety of disorders, including pregnancy loss, abnormal placental invasion (e.g., placenta accreta/percreta), preeclampsia, and intrauterine growth restriction. Improved understanding of complex maternal-fetal immune interactions will be crucial to discover mechanisms underpinning these pregnancy complications, which, in turn, will help inform preventative and/or therapeutic clinical interventions.
Similar content being viewed by others
References
Fuhler GM. The immune system and microbiome in pregnancy. Best Pract Res Clin Gastroenterol. 2020;101671:44–5. https://doi.org/10.1016/j.bpg.2020.101671.
Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol. 2001;158:1713–21. https://doi.org/10.1016/S0002-9440(10)64127-2.
Aisagbonhi O, Morris GP. Human leukocyte antigens in pregnancy and preeclampsia. Front Genet. 2022;13:884275. https://doi.org/10.3389/fgene.2022.884275.
Abril-Rodriguez G, Ribas A. SnapShot: immune checkpoint inhibitors. Cancer Cell. 2017;31(848–848).
Persson G, Jorgensen N, Nilsson LL, Andersen LHJ, Hviid TVF. A role for both HLA-F and HLA-G in reproduction and during pregnancy? Hum Immunol. 2020;81:127–33. https://doi.org/10.1016/j.humimm.2019.09.006.
Klein J, Sato A. The HLA system First of two parts. N Engl J Med. 2000;343:702–9. https://doi.org/10.1056/NEJM200009073431006.
Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA variation and disease. Nat Rev Immunol. 2018;18:325–39. https://doi.org/10.1038/nri.2017.143.
Cruz-Tapias P, Castiblanco, J, Anaya, J. Chapter 10: Major histocompatibility complex: Antigen processing and presentation. In: Anaya, JM, Shoenfeld, Y, Rojas-Villarraga, A, Levy, RA, & Cervera, R, editors. Autoimmunity: From Bench to Bedside (Internet); 2013.
Ferreira LMR, Meissner TB, Tilburgs T, Strominger JL. HLA-G: at the interface of maternal-fetal tolerance. Trends Immunol. 2017;38:272–86. https://doi.org/10.1016/j.it.2017.01.009.
Lin XX, Xie YM, Zhao SJ, Liu CY, Mor G, Liao AH. Human leukocyte antigens: the unique expression in trophoblasts and their crosstalk with local immune cells. Int J Biol Sci. 2022;18:4043–52. https://doi.org/10.7150/ijbs.73616.
Aptsiauri N, Garrido F. The challenges of HLA class I loss in cancer immunotherapy: facts and hopes. Clin Cancer Res. 2022;28:5021–9. https://doi.org/10.1158/1078-0432.CCR-21-3501.
Arnaiz-Villena A, et al. HLA-G: function, polymorphisms and pathology. Int J Immunogenet. 2021;48:172–92. https://doi.org/10.1111/iji.12513.
Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. 1999;189:1093–100. https://doi.org/10.1084/jem.189.7.1093.
Gonzalez A, Rebmann V, LeMaoult J, Horn PA, Carosella ED, Alegre E. The immunosuppressive molecule HLA-G and its clinical implications. Crit Rev Clin Lab Sci. 2012;49:63–84. https://doi.org/10.3109/10408363.2012.677947.
Xu X, Zhou Y, Wei H. Roles of HLA-G in the maternal-fetal immune microenvironment. Front Immunol. 2020;11:592010. https://doi.org/10.3389/fimmu.2020.592010.
Nilsson LL, Hviid TVF. HLA class Ib-receptor interactions during embryo implantation and early pregnancy. Hum Reprod Update. 2022;28:435–54. https://doi.org/10.1093/humupd/dmac007.
Rajagopalan S, Long EO. KIR2DL4 (CD158d): an activation receptor for HLA-G. Front Immunol. 2012;3:258. https://doi.org/10.3389/fimmu.2012.00258.
Rajagopalan S, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. https://doi.org/10.1371/journal.pbio.0040009.
Zhuang B, Shang J, Yao Y. HLA-G: an important mediator of maternal-fetal immune-tolerance. Front Immunol. 2021;12:744324. https://doi.org/10.3389/fimmu.2021.744324.
Krop J, Heidt S, Claas FHJ, Eikmans M. Regulatory T cells in pregnancy: it is not all about FOXP3. Front Immunol. 2020;11:1182. https://doi.org/10.3389/fimmu.2020.01182.
Tilburgs T, et al. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. 2009;82:148–57. https://doi.org/10.1016/j.jri.2009.05.003.
Svensson-Arvelund J, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015;194:1534–44. https://doi.org/10.4049/jimmunol.1401536.
Goodridge JP, Burian A, Lee N, Geraghty DE. HLA-F and MHC class I open conformers are ligands for NK cell Ig-like receptors. J Immunol. 2013;191:3553–62. https://doi.org/10.4049/jimmunol.1300081.
Jiang L, et al. Extracellular vesicle-mediated secretion of HLA-E by trophoblasts maintains pregnancy by regulating the metabolism of decidual NK cells. Int J Biol Sci. 2021;17:4377–95. https://doi.org/10.7150/ijbs.63390.
Papuchova H, Meissner TB, Li Q, Strominger JL, Tilburgs T. The dual role of HLA-C in tolerance and immunity at the maternal-fetal interface. Front Immunol. 2019;10:2730. https://doi.org/10.3389/fimmu.2019.02730.
Lashley LE, Haasnoot GW, Spruyt-Gerritse M, Claas FH. Selective advantage of HLA matching in successful uncomplicated oocyte donation pregnancies. J Reprod Immunol. 2015;112:29–33. https://doi.org/10.1016/j.jri.2015.05.006.
Meuleman T, Haasnoot GW, van Lith JMM, Verduijn W, Bloemenkamp KWM, Claas FHJ. Paternal HLA-C is a risk factor in unexplained recurrent miscarriage. Am J Reprod Immunol. 2018;79(2). https://doi.org/10.1111/aji.12797.
Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6:223. https://doi.org/10.1186/gb-2005-6-6-223.
Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. https://doi.org/10.1146/annurev.immunol.23.021704.115611.
Zhao Y, Zheng Q, Jin L. The role of B7 family molecules in maternal-fetal immunity. Front Immunol. 2020;11:458. https://doi.org/10.3389/fimmu.2020.00458.
Pulanco MC, Madsen AT, Tanwar A, Corrigan DT, Zang X. Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies. Cell Mol Immunol. 2023;20(7):694–713. https://doi.org/10.1038/s41423-023-01019-8.
Veras E, Kurman RJ, Wang TL, Shih IM. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol. 2017;36:146–53. https://doi.org/10.1097/pgp.0000000000000305.
Petroff MG, Kharatyan E, Torry DS, Holets L. The immunomodulatory proteins B7-DC, B7–H2, and B7–H3 are differentially expressed across gestation in the human placenta. Am J Pathol. 2005;167:465–73. https://doi.org/10.1016/s0002-9440(10)62990-2.
Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. https://doi.org/10.1038/85330.
Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550. https://doi.org/10.3389/fimmu.2016.00550.
Karvas RM, et al. Use of a human embryonic stem cell model to discover GABRP, WFDC2, VTCN1 and ACTC1 as markers of early first trimester human trophoblast. Mol Hum Reprod. 2020;26:425–40. https://doi.org/10.1093/molehr/gaaa029.
Galazka K, et al. Changes in the subpopulation of CD25+ CD4+ and FOXP3+ regulatory T cells in decidua with respect to the progression of labor at term and the lack of analogical changes in the subpopulation of suppressive B7–H4 macrophages–a preliminary report. Am J Reprod Immunol. 2009;61:136–46. https://doi.org/10.1111/j.1600-0897.2008.00674.x.
Zhou J, et al. The immune checkpoint molecule, VTCN1/B7-H4, guides differentiation and suppresses proinflammatory responses and MHC class I expression in an embryonic stem cell-derived model of human trophoblast. Front Endocrinol (Lausanne). 2023;14:1069395. https://doi.org/10.3389/fendo.2023.1069395.
Löhning M, et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–93. https://doi.org/10.1084/jem.20020632.
McAdam AJ, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–40. https://doi.org/10.4049/jimmunol.165.9.5035.
Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T Helper (Th) Cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front Immunol. 2020;11:2025. https://doi.org/10.3389/fimmu.2020.02025.
Chapoval AI, et al. B7–H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74. https://doi.org/10.1038/85339.
Chen C, Shen Y, Qu QX, Chen XQ, Zhang XG, Huang JA. Induced expression of B7–H3 on the lung cancer cells and macrophages suppresses T-cell mediating anti-tumor immune response. Exp Cell Res. 2013;319:96–102. https://doi.org/10.1016/j.yexcr.2012.09.006.
Li Y, et al. B7–H3 promotes gastric cancer cell migration and invasion. Oncotarget. 2017;8:71725–35. https://doi.org/10.18632/oncotarget.17847.
Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–92. https://doi.org/10.1084/jem.20100619.
Villarroel-Espindola F, et al. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin Cancer Res. 2018;24:1562–73. https://doi.org/10.1158/1078-0432.Ccr-17-2542.
Deng J, et al. Hypoxia-induced VISTA promotes the suppressive function of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Immunol Res. 2019;7:1079–90. https://doi.org/10.1158/2326-6066.Cir-18-0507.
Zong L, Zhang M, Wang W, Wan X, Yang J, Xiang Y. PD-L1, B7–H3 and VISTA are highly expressed in gestational trophoblastic neoplasia. Histopathology. 2019;75:421–30. https://doi.org/10.1111/his.13882.
Zhao SJ, Muyayalo KP, Luo J, Huang D, Mor G, Liao AH. Next generation of immune checkpoint molecules in maternal-fetal immunity. Immunol Rev. 2022;308:40–54. https://doi.org/10.1111/imr.13073.
Mellor AL, Munn DH. Extinguishing maternal immune responses during pregnancy: implications for immunosuppression. Semin Immunol. 2001;13:213–8. https://doi.org/10.1006/smim.2000.0317.
Mackler AM, Barber EM, Takikawa O, Pollard JW. Indoleamine 2,3-dioxygenase is regulated by IFN-gamma in the mouse placenta during Listeria monocytogenes infection. J Immunol. 2003;170:823–30. https://doi.org/10.4049/jimmunol.170.2.823.
Repnik U, et al. Comparison of macrophage phenotype between decidua basalis and decidua parietalis by flow cytometry. Placenta. 2008;29:405–12. https://doi.org/10.1016/j.placenta.2008.02.004.
Stammers M, Rowen L, Rhodes D, Trowsdale J, Beck S. BTL-II: a polymorphic locus with homology to the butyrophilin gene family, located at the border of the major histocompatibility complex class II and class III regions in human and mouse. Immunogenetics. 2000;51:373–82. https://doi.org/10.1007/s002510050633.
Nguyen T, Liu XK, Zhang Y, Dong C. BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation. J Immunol. 2006;176:7354–60. https://doi.org/10.4049/jimmunol.176.12.7354.
Swanson RM, et al. Butyrophilin-like 2 modulates B7 costimulation to induce Foxp3 expression and regulatory T cell development in mature T cells. J Immunol. 2013;190:2027–35. https://doi.org/10.4049/jimmunol.1201760.
Cui C, et al. In vivo administration of recombinant BTNL2-Fc fusion protein ameliorates graft-versus-host disease in mice. Cell Immunol. 2019;335:22–9. https://doi.org/10.1016/j.cellimm.2018.10.008.
Rieder SA, et al. B7–H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell Mol Immunol. 2021;18:1503–11. https://doi.org/10.1038/s41423-020-0361-7.
Zhu Z, et al. B7H6 serves as a negative prognostic marker and an immune modulator in human pancreatic cancer. Front Oncol. 2022;12:814312. https://doi.org/10.3389/fonc.2022.814312.
Zhao R, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci USA. 2013;110:9879–84. https://doi.org/10.1073/pnas.1303524110.
Enninga EAL, et al. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am J Reprod Immunol. 2018;79: e12795.
Mach P, et al. Changes in the blood serum levels of the costimulatory soluble B7–H4 molecule in pregnant women during the peripartal phase. Am J Reprod Immunol. 2015;74:209–15. https://doi.org/10.1111/aji.12392.
Mach P, et al. Serum concentrations of soluble B7–H4 in early pregnancy are elevated in women with preterm premature rupture of fetal membranes. Am J Reprod Immunol. 2016;76:149–54. https://doi.org/10.1111/aji.12527.
Mach P, et al. Soluble B7–H4 blood serum levels are elevated in women at high risk for preeclampsia in the first trimester, as well as in patients with confirmed preeclampsia. Am J Reprod Immunol. 2018;80:e12988. https://doi.org/10.1111/aji.12988.
Enninga EAL, et al. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am J Reprod Immunol. 2018;79(2):e12795. https://doi.org/10.1111/aji.12795
Mach P, Kimmig R, Kasimir-Bauer S, Buderath P. Association of soluble B7–H4 and circulating tumor cells in blood of advanced epithelial ovarian cancer patients. Front Oncol. 2021;11:721067. https://doi.org/10.3389/fonc.2021.721067.
Zhu X, Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget. 2017;8:97671. https://doi.org/10.18632/oncotarget.18311.
Frigola X, et al. Identification of a soluble form of B7–H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–23. https://doi.org/10.1158/1078-0432.CCR-10-0250.
Vargas A, et al. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. Faseb j. 2014;28:3703–19. https://doi.org/10.1096/fj.13-239053.
Chen G, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–6. https://doi.org/10.1038/s41586-018-0392-8.
Wu R, et al. Exosomal B7–H3 facilitates colorectal cancer angiogenesis and metastasis through AKT1/mTOR/VEGFA pathway. Cell Signal. 2023;109:110737. https://doi.org/10.1016/j.cellsig.2023.110737.
Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–20. https://doi.org/10.1038/nrd870.
Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–43. https://doi.org/10.1016/j.it.2012.10.001.
Fallarino F, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. https://doi.org/10.1038/sj.cdd.4401073.
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8. https://doi.org/10.4049/jimmunol.0903670.
Kudo Y, et al. Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta. J Reprod Immunol. 2004;61:87–98. https://doi.org/10.1016/j.jri.2003.11.004.
Kudo Y, et al. Mechanisms regulating the expression of indoleamine 2,3-dioxygenase during decidualization of human endometrium. Hum Reprod. 2004;19:1222–30. https://doi.org/10.1093/humrep/deh218.
Wei H, et al. Abnormal expression of indoleamine 2, 3-dioxygenase in human recurrent miscarriage. Reprod Sci. 2019 Mar 4;1933719119833788. https://doi.org/10.1177/1933719119833788
Iwahashi N, Yamamoto M, Nanjo S, Toujima S, Minami S, Ino K. Downregulation of indoleamine 2, 3-dioxygenase expression in the villous stromal endothelial cells of placentas with preeclampsia. J Reprod Immunol. 2017;119:54–60. https://doi.org/10.1016/j.jri.2017.01.003.
Munn DH, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. https://doi.org/10.1126/science.281.5380.1191.
Adams O, Besken K, Oberdorfer C, MacKenzie CR, Takikawa O, Daubener W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J Virol. 2004;78:2632–6. https://doi.org/10.1128/jvi.78.5.2632-2636.2004.
Popov A, et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J Clin Invest. 2006;116:3160–70. https://doi.org/10.1172/JCI28996.
Knubel CP, et al. Indoleamine 2,3-dioxigenase (IDO) is critical for host resistance against Trypanosoma cruzi. FASEB J. 2010;24:2689–701. https://doi.org/10.1096/fj.09-150920.
Kamimura S, Eguchi K, Yonezawa M, Sekiba K. Localization and developmental change of indoleamine 2 3-dioxygenase activity in the human placenta. Acta Med Okayama. 1991;45:135–9. https://doi.org/10.18926/AMO/32206.
Sedlmayr P. Indoleamine 2,3-dioxygenase in materno-fetal interaction. Curr Drug Metab. 2007;8:205–8. https://doi.org/10.2174/138920007780362491.
Kudo Y. The role of placental indoleamine 2,3-dioxygenase in human pregnancy. Obstet Gynecol Sci. 2013;56:209–16. https://doi.org/10.5468/ogs.2013.56.4.209.
Chang RQ, Li DJ, Li MQ. The role of indoleamine-2,3-dioxygenase in normal and pathological pregnancies. Am J Reprod Immunol. 2018;79:e12786. https://doi.org/10.1111/aji.12786.
Zardoya-Laguardia P, et al. Endothelial indoleamine 2,3-dioxygenase-1 regulates the placental vascular tone and is deficient in intrauterine growth restriction and pre-eclampsia. Sci Rep. 2018;8:5488. https://doi.org/10.1038/s41598-018-23896-0.
Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J Immunother Cancer. 2015;3:51. https://doi.org/10.1186/s40425-015-0094-9.
Tang K, Wu YH, Song Y, Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol. 2021;14:68. https://doi.org/10.1186/s13045-021-01080-8.
Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. https://doi.org/10.1146/annurev-immunol-032713-120142.
Takizawa H, Manz MG. Macrophage tolerance: CD47-SIRP-alpha-mediated signals matter. Nat Immunol. 2007;8:1287–9. https://doi.org/10.1038/ni1207-1287.
Chao MP, Weissman IL, Majeti R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–32. https://doi.org/10.1016/j.coi.2012.01.010.
Ide K, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104:5062–6. https://doi.org/10.1073/pnas.0609661104.
Kovacs AF, et al. The impact of circulating preeclampsia-associated extracellular vesicles on the migratory activity and phenotype of THP-1 monocytic cells. Sci Rep. 2018;8:5426. https://doi.org/10.1038/s41598-018-23706-7.
Tong M, et al. Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: relevance for feto-maternal communication. Hum Reprod. 2016;31:687–99. https://doi.org/10.1093/humrep/dew004.
Liu X, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–15. https://doi.org/10.1038/nm.3931.
Vonderheide RH. CD47 blockade as another immune checkpoint therapy for cancer. Nat Med. 2015;21:1122–3. https://doi.org/10.1038/nm.3965.
Funding
The authors’ research is supported by grants 1RO1HD094937 from the National Institutes of Health (DJS), R21A1145071 from the National Institutes of Health (DJS, JZ), and pilot grant from the University of Missouri (SC).
Author information
Authors and Affiliations
Contributions
S.M., J.Z., and S.C. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mebane, S., Zhou, J., Choi, S. et al. Immune Checkpoint Molecules and Maternal–Fetal Immunity. Curr Obstet Gynecol Rep 13, 37–45 (2024). https://doi.org/10.1007/s13669-024-00372-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-024-00372-3