Abstract
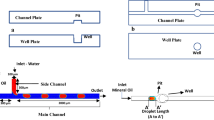
The elegance of digital microfluidics is in incorporating high quantities of manipulated micro and nanodroplets on-chip, each of which can be considered a small-volume carrier of various chemical and biological reagents. Therefore, the analysis of on-demand manipulation of these micro and nanocarriers is extremely important in developing an optimized lab on a chip. In this work, passive coalescence and mixing between two trapped, squeezed nanodroplets inside a closed microfluidic device was investigated. The droplets are composed of glycerol dyed in blue and red, dispersed inside oleic acid as the carrier oil. A microwell with a circular cross section was fabricated on the top wall of the microchannels to trap the first droplet for increasing the mixing precision and minimizing the viscous shear stress imposed on the droplets from the channel walls. The energy minimization theory was used to develop a parametric study for this trapping technique and to choose the optimum design parameters for droplet trapping in terms of efficiency. Image processing was performed on the snapshots of the trapped glycerol nanodroplets during mixing. Growth in passive mixing percentage was demonstrated to be asymptotical and was formulated with an empirical equation of exponential form as a function of the passive mixing relaxation time. The required time for the passive mixing of a glycerol droplet pair was measured considering various thresholds for the final standard deviation of the gray intensity indices. This finding was of the order of magnitude of the diffusive mixing time scale and physically consistent with the Stokes flow regime.
Similar content being viewed by others
Change history
05 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s42757-022-0132-z
References
Abbyad, P., Dangla, R., Alexandrou, A., Baroud, C. N. 2011. Rails and anchors: Guiding and trapping droplet microreactors in two dimensions. Lab Chip, 11: 813–821.
Bamshad, A., Nikfarjam, A., Sabour, M. H. 2018. Capillary-based microoptofluidic viscometer. Meas Sci Technol, 29: 095901.
Bamshad, A., Nikfarjam, A., Sabour, M. H., Raji, H. 2017. Theoretical and numerical investigation of liquid-gas interface location of capillary driven flow during the time throughout circular microchannels. In: Proceedings of the 5th RSI International Conference on Robotics and Mechatronics, 432–438.
Bithi, S. S., Vanapalli, S. A. 2010. Behavior of a train of droplets in a fluidic network with hydrodynamic traps. Biomicrofluidics, 4: 044110.
Bithi, S. S., Wang, W. S., Sun, M., Blawzdziewicz, J., Vanapalli, S. A. 2014. Coalescing drops in microfluidic parking networks: A multifunctional platform for drop-based microfluidics. Biomicrofluidics, 8: 034118.
Boukellal, H., Selimović, Š., Jia, Y. W., Cristobal, G., Fraden, S. 2009. Simple, robust storage of drops and fluids in a microfluidic device. Lab Chip, 9: 331–338.
Bringer, M. R., Gerdts, C. J., Song, H., Tice, J. D., Ismagilov, R. F. 2004. Microfluidic systems for chemical kinetics that rely on chaotic mixing in droplets. Philos T Roy Soc A, 362: 1087–1104.
Chen, H., Fang, Q., Yin, X. F., Fang, Z. L. 2005. Microfluidic chip-based liquid-liquid extraction and preconcentration using a subnanoliter-droplet trapping technique. Lab Chip, 5: 719–725.
Dangla, R., Lee, S., Baroud, C. N. 2011. Trapping microfluidic drops in wells of surface energy. Phys Rev Lett, 107: 124501.
Karbalaei, A., Cho, H. J. 2018. Microfluidic devices developed for and inspired by thermotaxis and chemotaxis. Micromachines, 9: 149.
Karbalaei, A., Kumar, R., Cho, H. J. 2016. Thermocapillarity in microfluidics—A review. Micromachines, 7: 13.
Paik, P., Pamula, V. K., Pollack, M. G., Fair, R. B. 2003. Electrowetting-based droplet mixers for microfluidic systems. Lab Chip 3: 28–33.
Schneider, C. A., Rasband, W. S., Eliceiri, K. W. 2012. NIH image to ImageJ: 25 years of image analysis. Nat Methods, 9: 671–675.
Song, H., Bringer, M. R., Tice, J. D., Gerdts, C. J., Ismagilov, R. F. 2003. Experimental test of scaling of mixing by chaotic advection in droplets moving through microfluidic channels. Appl Phys Lett, 83: 4664–4666.
Tan, W. H., Takeuchi, S. 2007. A trap-and-release integrated microfluidic system for dynamic microarray applications. P Natl Acad Sci USA, 104: 1146–1151.
Tice, J. D., Lyon, A. D., Ismagilov, R. F. 2004. Effects of viscosity on droplet formation and mixing in microfluidic channels. Anal Chim Acta, 507: 73–77.
Tice, J. D., Song, H., Lyon, A. D., Ismagilov, R. F. 2003. Formation of droplets and mixing in multiphase microfluidics at low values of the Reynolds and the capillary numbers. Langmuir, 19: 9127–9133.
Wang, W., Yang, C., Li, C. M. 2009. On-demand microfluidic droplet trapping and fusion for on-chip static droplet assays. Lab Chip, 9: 1504–1506.
Acknowledgements
Authors thank Saleheh Seif for guidance on image processing and statistical analysis and Lucille Serody for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karbalaei, A., Cho, H.J. Passive mixing rate of trapped squeezed nanodroplets—A time scale analysis. Exp. Comput. Multiph. Flow 2, 135–141 (2020). https://doi.org/10.1007/s42757-019-0044-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42757-019-0044-8