Abstract
Background
The administration of antiplatelet drugs before coronary artery bypass graft surgery (CABG) is associated with an increased risk of major hemorrhage and related surgical reexploration. Little is known about the relative effect of combined clopidogrel and aspirin on blood product use around the time of CABG. We evaluated the associated risk between the combined use of aspirin and clopidogrel and the transfusion of blood products perioperatively.
Methods
We retrospectively studied a cohort of 659 individuals who underwent a first CABG, without concomitant valvular or aortic surgery, at a single large Canadian cardiac surgical centre between January 2000 and April 2002. The four study exposure groups were those prescribed aspirin (n = 105), clopidogrel (n = 11), the combination of both (n = 46), or neither drug (n = 497), within 7 days prior to CABG. The primary study outcome was the excessive transfusion of blood products during CABG and up to the second post-operative day, defined as ≥ 2 units of packed red blood cells (PRBC), ≥ 2 units of fresh frozen plasma, ≥ 5 units of cryoprecipitate or ≥ 5 units of platelets. Secondary outcomes included the mean number of transfused units of each type of blood product.
Results
A greater mean number of units of PRBC were transfused among those who received clopidogrel alone (2.9) or in combination with aspirin (2.4), compared to those on aspirin alone (1.9) or neither antiplatelet drug (1.4) (P = 0.001). A similar trend was seen for the respective mean number of transfused units of platelets (3.6, 3.7, 1.3 and 1.0; P < 0.001) and fresh frozen plasma (2.5, 3.1, 2.3, 1.6; P = 0.01). Compared to non-users, the associated risk of excessive blood product transfusion was highest among recipients of aspirin and clopidogrel together (adjusted OR 2.2, 95% CI 1.1–4.3). No significant association was seen among lone users of aspirin (adjusted OR 1.0, 95% CI 0.6–1.6) or clopidogrel (adjusted OR 0.7, 95% CI 0.2–2.5), compared to non-users.
Conclusions
While combined use of aspirin and clopidogrel shortly before CABG surgery may increase the associated risk of excess transfusion of blood products perioperatively, several study limitations prevent any confident conclusions from being drawn. Beyond challenging these findings, future research might focus on the value of both intraoperative monitoring of platelet function, and the effectiveness of antifibrinolytic agents, at reducing the risk of postoperative bleeding.
Similar content being viewed by others
Background
Antiplatelet agents are effective in the management of stable and unstable coronary artery disease [1]. Early initiation of these drugs also reduces the risk of graft occlusions after coronary artery bypass surgery (CABG) [2]. The combination of clopidogrel, an adenosine diphosphate receptor (ADP) antagonist, and aspirin, has been shown to reduce the risk of cardiovascular death or nonfatal myocardial infarction among individuals presenting with non-STEMI acute coronary syndromes [3]. A similar benefit was seen among those receiving percutaneous coronary interventions [4].
The potent inhibition of platelet function achieved by the combination of aspirin and clopidogrel leads to a prolongation of the bleeding time [5] and an increased risk of major hemorrhage [3], thus, raising concerns about their safety around the time of CABG [6]. In one small study, clopidogrel and aspirin use before CABG was associated with more bleeding complications and blood product transfusions [7]. In the current study, we investigated whether recent use of aspirin, clopidogrel, or both was associated with an increased risk of blood product transfusion during and soon after CABG.
Methods
We conducted a retrospective cohort study at the Hamilton Health Sciences Corporation, General Division, a large academic referral centre for patients from Southwestern Ontario. The immediate postoperative care of these patients is provided through a dedicated cardiac surgery intensive care unit (ICU), staffed by trained intensivists and nurses. Pericardial and pleural chest tube output is monitored frequently within the first few hours after surgery, and then hourly thereafter. Once stable, extubated patients are transferred to a cardiac surgery step-down unit, on at least the second postoperative day. The use of intraoperative blood products or antifibrinolytic agents (e.g., aprotinin) is left to the discretion of the anesthetist and surgeon, while postoperative transfusions are usually decided upon by the attending intensivist.
All laboratory results and blood product transfusion information are entered into the hospital's patient care computer system from the respective laboratories. Using the patient hospital identification number, information is available about the type of blood product transfused, the number of units and volume, and the date of transfusion. This same computer system also contains a complete medication history for each patient, including the drug name, dose, and date of initiation and cessation.
We included patients who underwent their first CABG, without concomitant valvular or aortic surgery, between January 2000 and April 2002. A total of 1626 individuals were identified from a regularly updated register of all CABG recipients at our centre. A list of randomly generated numbers was created in Excel 5.0 (Microsoft Corporation), merged alongside the original list of CABG patients, and then randomly re-sorted once more. From this new list of 1626 randomly sorted patients we abstracted data on 675 patients, surpassing the estimated sample size, as outlined below. Of these 675 individuals, the identification numbers of 7 did not match with those in the patient care computer system, while another 9 did not undergo isolated CABG surgery. Thus, a total of 659 patients were included herein.
The four study exposure groups were divided into those prescribed i) aspirin, ii) clopidogrel, iii) both drugs or iv) neither drug, each ≤ 7 days before CABG. The primary study outcome was the transfusion of an excessive number of blood products during, and up to, the second post-CABG day. The latter was defined as ≥ 2 units of non-autologous packed red blood cells (PRBC), ≥ 2 units of fresh frozen plasma (FFP), ≥ 5 units of cryoprecipitate, or ≥ 5 units of platelets – quantities that are both clinically important and financially costly [8, 9]. Other secondary outcomes included: i) the transfusion of any quantity of blood products up to the second post-CABG day; ii) the mean number of transfused units of each type of blood product, up to the second post-operative day; iii) the mean number of transfused units of FFP or cryoprecipitate; and iv) the mean number of transfused units of FFP, cryoprecipitate or platelets, together a reflection of a patient's perceived or actual bleeding tendency.
This study was solely designed to examine blood product use among a large sample of patients. Accordingly, we used the hospital computer system as the single source of all study data; no information was obtained about preoperative renal function, post-operative chest tube drainage, hemodynamics, hematological indices, or the need for surgical reexploration. Whether the surgery was urgent or elective was not recorded, nor were other patient characteristics, including comorbidity factors.
Statistical analysis
We expressed the risk of excessive transfusion in relation to antiplatelet drug use (i.e., no drug, aspirin, clopidogrel or both), using unadjusted and adjusted odds ratios (OR) and a 95% confidence interval (CI). With logistic regression analysis, we adjusted for participant age, sex and receipt of any autologous whole blood within 2 days following surgery. Patient characteristics and study outcomes were compared between the four drug exposure groups using ANOVA for continuous variables, and a chi-square test for categorical data. The study sample size was estimated assuming that, for each person receiving combined aspirin and clopidogrel, at least 6 patients would receive neither drug. Expecting that 50% of non-users and 70% of combined users might receive an excessive amount of blood products [7], and setting α = 0.05 and β = 0.20, we estimated that approximately 50 combined users and 325 non-users would be needed.
Statistical significance was set at a 2-sided P-value of 0.05, and all analyses were performed using SAS Version 8.0 (SAS Institute Inc., Cary, North Carolina). The Hamilton Health Sciences Corporation Research Ethics Board granted permission to conduct this audit study.
Results
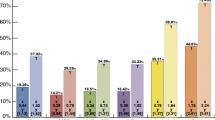
A total of 105 individuals (16.0%) received aspirin alone, 11 (1.7%) received clopidogrel alone, 46 (7.0%) received aspirin and clopidogrel, while the remaining 497 patients (75%) did not receive an antiplatelet drug before surgery (Table 1). Neither the mean age nor preoperative hemoglobin concentration was significantly different between groups, but there were more women prescribed clopidogrel. Those not prescribed an antiplatelet drug were more likely to have received autologous whole blood (P = 0.01) (Table 1).
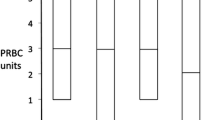
Individuals prescribed either clopidogrel, or clopidogrel plus aspirin, were transfused more blood products during and early after surgery, compared to those prescribed neither (Table 2). On average, 2.9 units of PRBC were given to clopidogrel users, 2.4 units to combined users, and only 1.4 units to non-users (P = 0.001). Those prescribed clopidogrel (3.6 units) or clopidogrel plus aspirin (3.7 units) received more platelets than those prescribed aspirin (1.3 units) or neither drug (1.0 units) (Table 2). Clopidogrel users, either alone (8.0 units), or in combination with aspirin (7.5 units), received almost twice the total number of units of FFP, cryoprecipitate and platelets, than those on aspirin (4.2 units) or neither drug (3.3 units) (P = 0.006) (Table 2).
Compared to non-users, the risk of excessive blood product transfusion was highest among recipients of combined aspirin and clopidogrel (crude OR 2.3, 95% CI 1.2–4.5), an effect that persisted after adjustment for a few potential confounders (adjusted OR 2.2, 95% CI 1.1–4.3). No increased risk was seen among users of aspirin (adjusted OR 1.0, 95% CI 0.6–1.6) or clopidogrel alone (adjusted OR 0.7, 95% CI 0.2–2.5) (Table 3). Similarly, compared to non-users, those recently prescribed combined aspirin and clopidogrel were more likely to receive 1 or more units of any type of blood product (crude OR 2.4, 95% CI 1.2–4.9). This risk estimate was further attenuated after adjusting for age, sex and autologous whole blood use (adjusted OR 2.1, 95% CI 1.0–4.5) (Table 3).
Discussion
In a cohort of 659 CABG patients, we observed an increased total volume and associated risk of blood product transfusion among those recently prescribed preoperative clopidogrel in combination with aspirin.
We did not obtain information about patient comorbidities (e.g., recent myocardial infarction), whether the CABG was elective or emergent [10], or about the use of preoperative anticoagulants or thrombolytic drugs [11]. Unstable patients undergoing emergency CABG might be expected to receive dual antiplatelet therapy at higher rates than elective cases, and because they often require more intense perioperative support and monitoring [10], urgent cases might also receive more blood products. The relationship between dual antiplatelet drug use and perioperative blood product use is likely confounded by several elements, as noted by the decline in the OR for transfusion of any blood product from 2.4 to 2.1, after adjusting for age, sex and autologous whole blood use (Table 3). Since these data were derived from a patient care database, some individuals classified as not having received an antiplatelet drug may have actually received one or both drugs just prior to their hospital admission for elective CABG. Such misclassification bias usually leads to a diminution of the observed effect size, such that we may have underestimated the true association between dual antiplatelet drug use and excessive blood product transfusion.
Most surgeons and anesthetists likely knew whether their patient had recently received an antiplatelet drug, possibly lowering their threshold for administering blood products or antifibrinolytic agents to minimize bleeding. Furthermore, we did not assess for other clinical indicators of bleeding risk, such as preoperative renal function, postoperative chest tube output or hypotension in the ICU. Finally, because few patients received clopidogrel alone, confident conclusions cannot be drawn about their overall need for blood product transfusion. In light of these potential threats to validity, our data should be viewed as hypothesis generating; however, they may assist in designing future research studies, while raising appropriate questions about how to best minimize blood product use in the current era of combined antiplatelet drug therapy [3, 12].
These study findings, and those of others, imply that use of dual antiplatelet agents before CABG is associated with increased blood product transfusion among some patients, a reflection of post-operative bleeding risk. It has been suggested that this risk may be related to the timing of the last administered drug dose, a point not properly defined in our study. For example, in a prospective study of 100 CABG patients, those who received aspirin within 2 days of surgery had greater impairment of platelet function than participants who stopped their aspirin 3 to 7 days preoperatively [13]. A similar dose-dependent effect on bleeding time and ex vivo ADP-induced platelet aggregation has also been observed for clopidogrel [14].
In the CURE trial, 12,562 patients presenting with non-STEMI acute coronary syndromes were randomized to receive either aspirin plus clopidogrel or aspirin plus placebo [3]. Participants who required CABG, and who received dual antiplatelet therapy within 5 days prior to surgery, had a higher rate of major postoperative bleeding than those assigned aspirin and placebo (9.6% vs. 6.3%; P = 0.06) [3]. In a prospective cohort study of 247 patients undergoing CABG, 51 individuals received preoperative aspirin plus clopidogrel [7]. In the latter group, there was a higher rate of reoperation for bleeding compared to those prescribed aspirin alone (9.8% vs. 1.6%; OR 6.9, 95% CI 1.6–30). Moreover, recipients of both drugs were transfused more units of PRBC (3 vs. 1.6; P < 0.001), platelets (4.3 vs. 1.7; P = 0.05), FFP (1.1 vs. 0.6; P = 0.08) and cryoprecipitate (2.4 vs. 1.2; P = 0.04) [7].
In the most recently published cohort study, among the 59 patients who received clopidogrel up to 7 days before CABG, there was about 250 mL more of chest tube drainage 8 hours after surgery, compared to non-recipients [15]. Persons administered clopidogrel also had a higher rate of reoperation for bleeding (6.8% vs. 0.6%), and received more units of PRBC (2.5 vs. 1.7), platelets (0.9 vs. 0.2) and FFP (0.7 vs. 0.2) [15]. Unlike those prescribed aspirin alone, patients taking both aspirin and clopidogrel required a greater number of units of PRBC (2.6 vs. 1.6), platelets (0.9 vs. 0.2) and FFP (0.8 vs. 0.3), compared to individuals who received neither drug [15]. Together, these data suggest that recent administration of clopidogrel, especially in combination with aspirin, increases the risk of postoperative bleeding [16].
Deciding if, and when, antiplatelet agents should be held prior to CABG may depend not only on safety issues (e.g., postoperative bleeding risk), but also on maintaining graft patency [2]. After the first week of CABG, reactive thrombocytosis is common, and may be an important risk factor for late symptomatic vein graft occlusion [17]. In a case-control study of the 8,641 consecutive CABG procedures performed during the late 1980's and early 1990's, preoperative aspirin use was associated with a lower risk of in-hospital mortality, compared with drug cessation within 7 days before surgery (adjusted OR 0.55, 95% CI 0.31–0.98). Furthermore, there was no increased risk of major bleeding [18]. While similar data on graft patency are not available for clopidogrel, one small randomized double-masked placebo-controlled trial showed that initiation of ticlopidine (an older ADP inhibitor) on day 2 post-CABG, and continued for another 12 months, was associated with significantly lower rate of graft occlusion at up to 1 year [19]. Thus, until more data are made available, it would seem both safe and sensible to withhold clopidogrel, and perhaps, aspirin, for at least 5 days before CABG, and then to restart one or both drugs soon after surgery [2].
Since some individuals continue to receive dual antiplatelet drugs up to the time of CABG, often with emergency surgery, other methods should be considered to reduce the risk of major postoperative bleeding, including the use of antifibrinolytic agents. In one clinical trial, 202 CABG patients were randomized to receive either placebo or low-dose aprotinin, a serine protease inhibitor, at a dose of 0.5 million kallikrein inhibiting units (KIU) twice during surgery [20]. Aprotinin recipients required significantly fewer blood products, with the greatest benefit seen among those taking aspirin [20]. In a second double-masked clinical trial, 55 patients who were taking aspirin until the day before their CABG surgery were randomized to receive a single dose of either 2 million KIU of aprotinin or placebo at the time of sternal skin closure [21]. Aprotinin recipients had a significantly lower hourly rate (28 vs. 43 mL/h), and total volume (955 vs. 1570 mL), of chest tube drainage, as well as a significant reduction in the transfusion of PRBC (47% reduction), platelets (77% reduction), FFP (88% reduction) and all blood products (68% reduction) [21]. Thus, balanced against the known cost, safety and availability of blood products [22] are the potentially beneficial, but expensive, drugs like aprotinin, and the less expensive agents, including tranexamic acid [8]. Perhaps by restricting their use to patients at high risk for major postoperative bleeding, including those taking combined aspirin and clopidogrel, the cost of these drugs can be better contained. Whether the use of intraoperative monitoring of platelet function [23] can further enhance this decision making process also remains unknown. A randomized clinical trial, with a formal cost-effectiveness analysis, might best address these points together.
Conclusions
The combined use of aspirin and clopidogrel shortly before CABG surgery may increase the associated risk of excess transfusion of blood products perioperatively. In lieu of several study limitations herein, more valid data are needed before the current findings are applied to clinical practice.
Author's Contributions
JR Designed the study, collected the data, performed the statistical analysis, and wrote the manuscript. SD Designed the study and collected the data. AO Designed the study and collected the data. EA Designed the study and collected the data. MV Designed the study and performed the statistical analysis. KA Designed the study and helped collect the data. DC Designed the study and helped collect the data. IC Designed the study, analyzed the data, and co-wrote the manuscript. CH Designed the study, helped collect the data, and co-wrote the manuscript.
All authors read and approved the final manuscript.
References
Antiplatelet Trialists' Collaboration: Collaborative overview of randomised trials of antiplatelet therapy-I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994, 308: 81-106.
Henderson WG, Goldman S, Copeland JG, Moritz TE, Harker LA: Antiplatelet or anticoagulant therapy after coronary artery bypass surgery. A meta-analysis of clinical trials. Ann Intern Med. 1989, 111: 743-50.
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK: Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001, 345: 494-502. 10.1056/NEJMoa010746.
Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA: Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001, 358: 527-33. 10.1016/S0140-6736(01)05701-4.
Payne DA, Hayes PD, Jones CI, Belham P, Naylor AR, Goodall AH: Combined therapy with clopidogrel and aspirin significantly increases the bleeding time through a synergistic antiplatelet action. J Vasc Surg. 2002, 35: 1204-9. 10.1067/mva.2002.122027.
D'Ancona G, Donias HW, Karamanoukian RL, Bergsland J, Karamanoukian HL: OPCAB therapy survey: off-pump clopidogrel, aspirin or both therapy survey. Heart Surg Forum. 2001, 4: 354-8.
Yende S, Wunderink RG: Effect of clopidogrel on bleeding after coronary artery bypass surgery. Crit Care Med. 2001, 29: 2271-5.
Laupacis A, Fergusson D: Drugs to minimize perioperative blood loss in cardiac surgery: meta-analyses using perioperative blood transfusion as the outcome. The International Study of Peri-operative Transfusion (ISPOT) Investigators. Anesth Analg. 1997, 85: 1258-67.
Hardy JF: Pharmacological strategies for blood conservation in cardiac surgery: erythropoietin and antifibrinolytics. Can J Anaesth. 2001, 48 (4 Suppl): 24-31.
Hochberg MS, Gregory JJ, McCullough J, Gielchinsky I, Hussain SM, Fuzesi L, Parsonnet V: Early emergent coronary bypass after failed angioplasty. N Engl J Med. 1993, 90: 385-91.
Grubitzsch H, Wollert HG, Eckel L: Emergency coronary artery bypass grafting: does excessive preoperative anticoagulation increase bleeding complications and transfusion requirements?. Cardiovasc Surg. 2001, 9: 510-6. 10.1016/S0967-2109(01)00049-7.
Nikhil JY, Radhakrishnan S, Paradiso-Hardy FL, Cohen EA: Clopidogrel in interventional cardiology: questions answered and questions remaining. Can J Cardiol. 2002, 18: 739-48.
Gibbs NM, Weightman WM, Thackray NM, Michalopoulos N, Weidmann C: The effects of recent aspirin ingestion on platelet function in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2001, 15: 55-9. 10.1053/jcan.2001.20277.
David JL, Limet R: Antiplatelet activity of clopidogrel in coronary artery bypass graft surgery patients. Thromb Haemost. 1999, 82: 1417-21.
Hongo RH, Ley J, Dick SE, Yee RR: The effect of clopidogrel in combination with aspirin when given before coronary artery bypass grafting. J Am Coll Cardiol. 2002, 40: 231-7. 10.1016/S0735-1097(02)01954-X.
Vuylsteke A, Oduro A, Cardan E, Latimer RD: Effect of aspirin in coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 1997, 11: 831-4.
Schmuziger M, Christenson JT, Maurice J, Simonet F, Velebit V: Reactive thrombocytosis after coronary bypass surgery. An important risk factor. Eur J Cardiothorac Surg. 1995, 9: 393-7.
Dacey LJ, Munoz JJ, Johnson ER, Leavitt BJ, Maloney CT, Morton JR, Olmstead EM, Birkmeyer JD, O'Connor GT: Effect of preoperative aspirin use on mortality in coronary artery bypass grafting patients. Ann Thorac Surg. 2000, 70: 1986-90. 10.1016/S0003-4975(00)02133-0.
Limet R, David JL, Magotteaux P, Larock MP, Rigo P: Prevention of aorta-coronary bypass graft occlusion. Beneficial effect of ticlopidine on early and late patency rates of venous coronary bypass grafts: a double-blind study. J Thorac Cardiovasc Surg. 1987, 94: 773-83.
Dignan RJ, Law DW, Seah PW, Manganas CW, Newman DC, Grant PW, Wolfenden HD: Ultra-low dose aprotinin decreases transfusion requirements and is cost effective in coronary operations. Ann Thorac Surg. 2001, 71: 158-63. 10.1016/S0003-4975(00)01860-9.
Alvarez JM, Jackson LR, Chatwin C, Smolich JJ: Low-dose postoperative aprotinin reduces mediastinal drainage and blood product use in patients undergoing primary coronary artery bypass grafting who are taking aspirin: a prospective, randomized, double-blind, placebo-controlled trial. J Thorac Cardiovasc Surg. 2001, 122: 457-63. 10.1067/mtc.2001.115701.
Hebert PC, Yetisir E, Martin C, Blajchman MA, Wells G, Marshall J, Tweeddale M, Pagliarello G, Schweitzer I: Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases?. Crit Care Med. 2001, 29: 227-34. 10.1097/00003246-200102000-00001.
Mukherjee D, Chew DP, Robbins M, Yadav JS, Raymond RE, Moliterno DJ: Clinical application of procedural platelet monitoring during percutaneous coronary intervention among patients at increased bleeding risk. J Thromb Thrombolysis. 2001, 11: 151-4. 10.1023/A:1011228817265.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2261/3/3/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing Interests
None declared.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ray, J.G., Deniz, S., Olivieri, A. et al. Increased blood product use among coronary artery bypass patients prescribed preoperative aspirin and clopidogrel. BMC Cardiovasc Disord 3, 3 (2003). https://doi.org/10.1186/1471-2261-3-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2261-3-3